Nat Prod Sci.
2017 Jun;23(2):75-83. 10.20307/nps.2017.23.2.75.
Microwave Assisted Extraction, Optimization using Central Composite Design, Quantitative Estimation of Arjunic Acid and Arjunolic Acid using HPTLC and Evaluation of Radical Scavenging Potential of Stem Bark of Terminalia arjuna
- Affiliations
-
- 1Vaish Institute of Pharmaceutical Education and Research, Rohtak, India.
- 2Faculty of Pharmaceutical Sciences, M.D. University, Rohtak, India. an_mdu@rediffmail.com
- 3Faculty of Pharmacy, Jamia Hamdard, New Delhi, India.
- KMID: 2387055
- DOI: http://doi.org/10.20307/nps.2017.23.2.75
Abstract
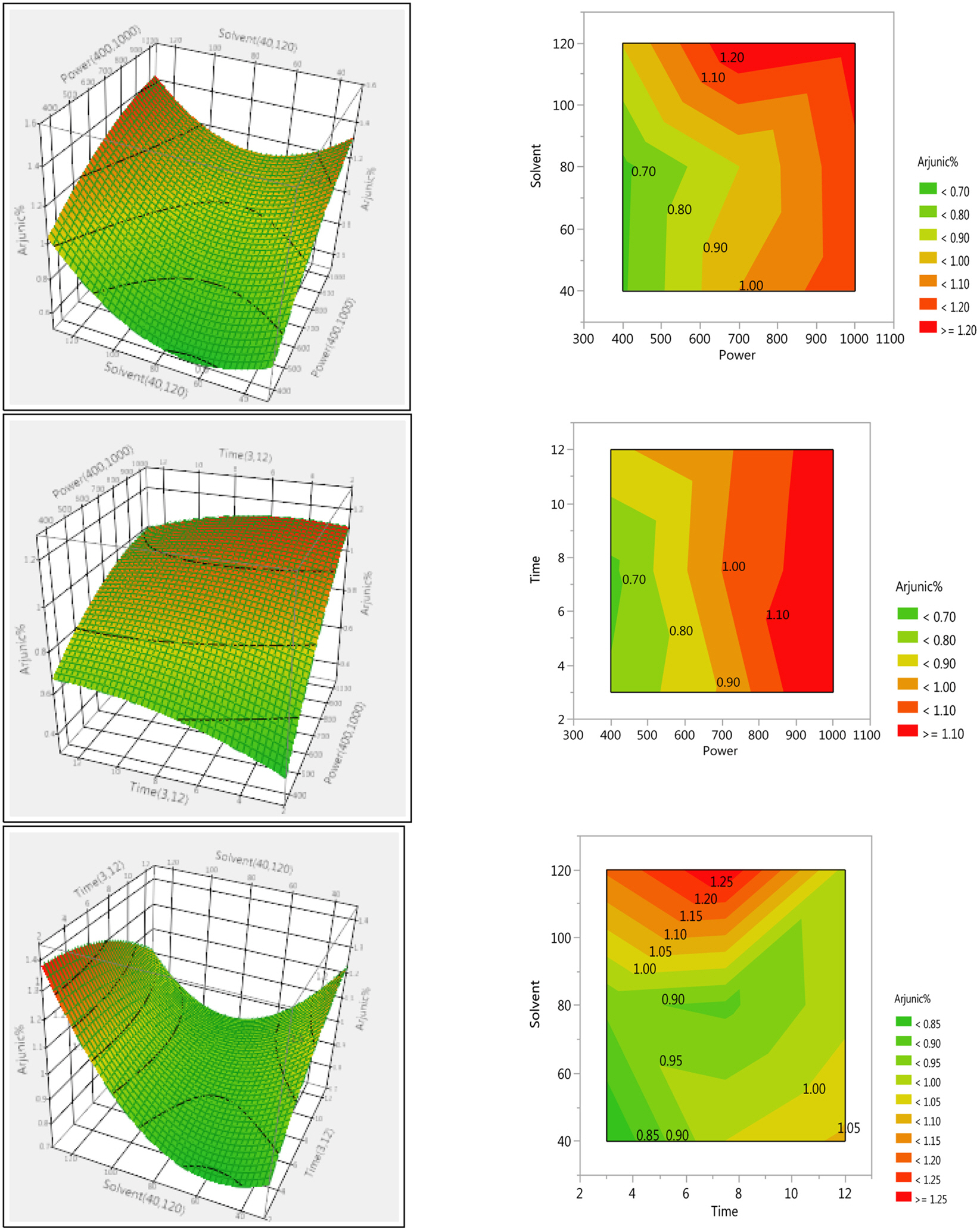
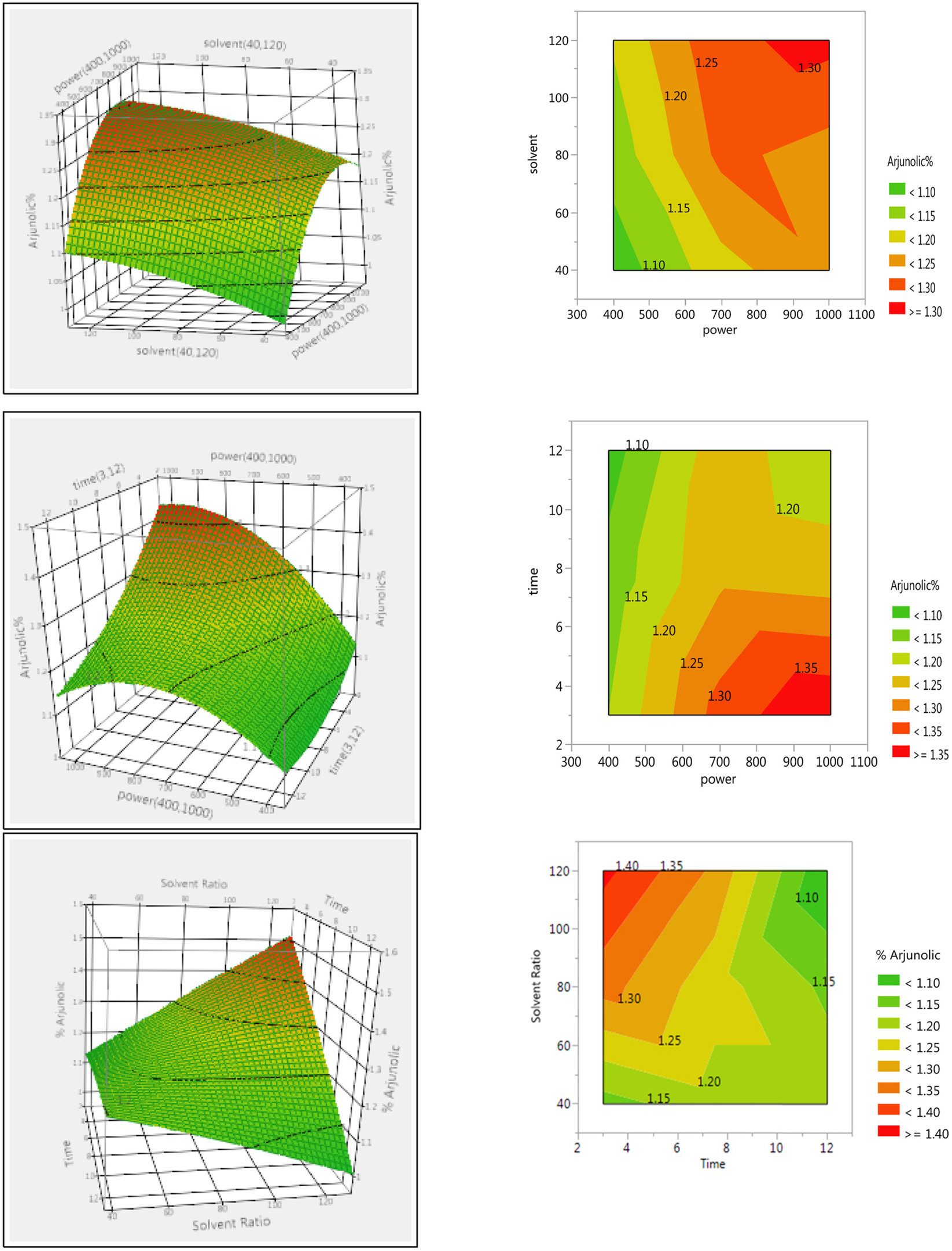
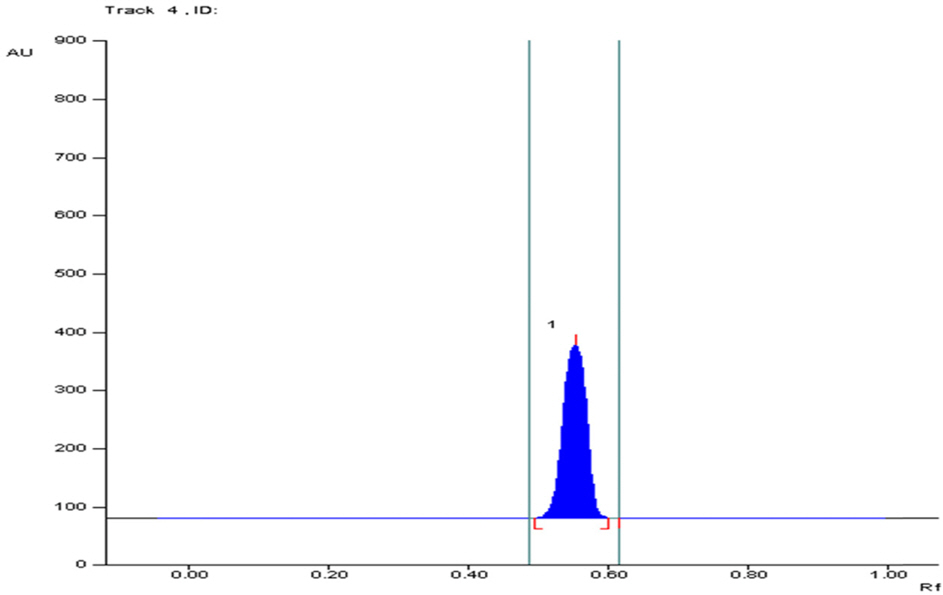
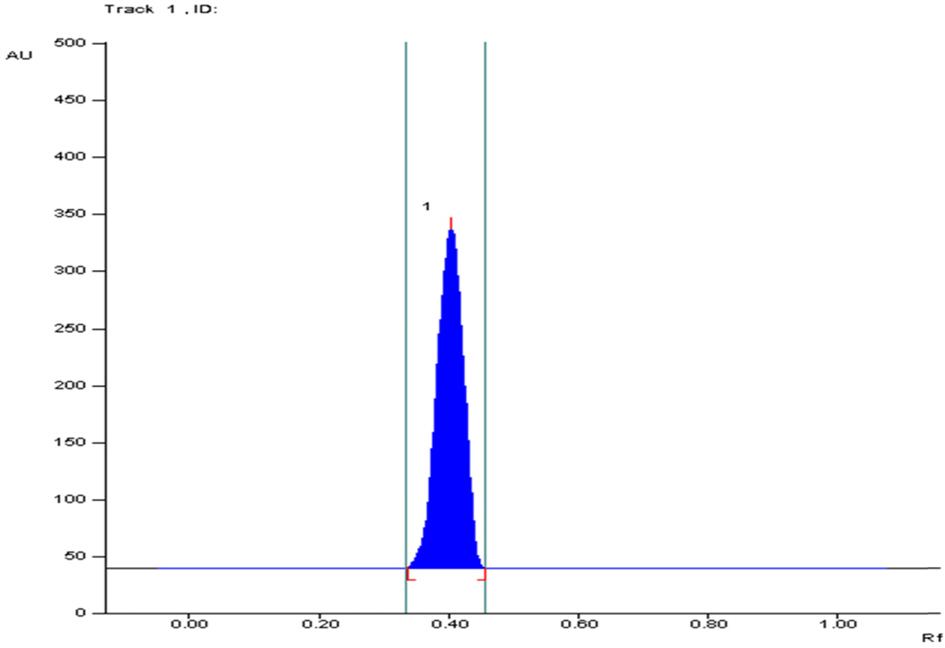
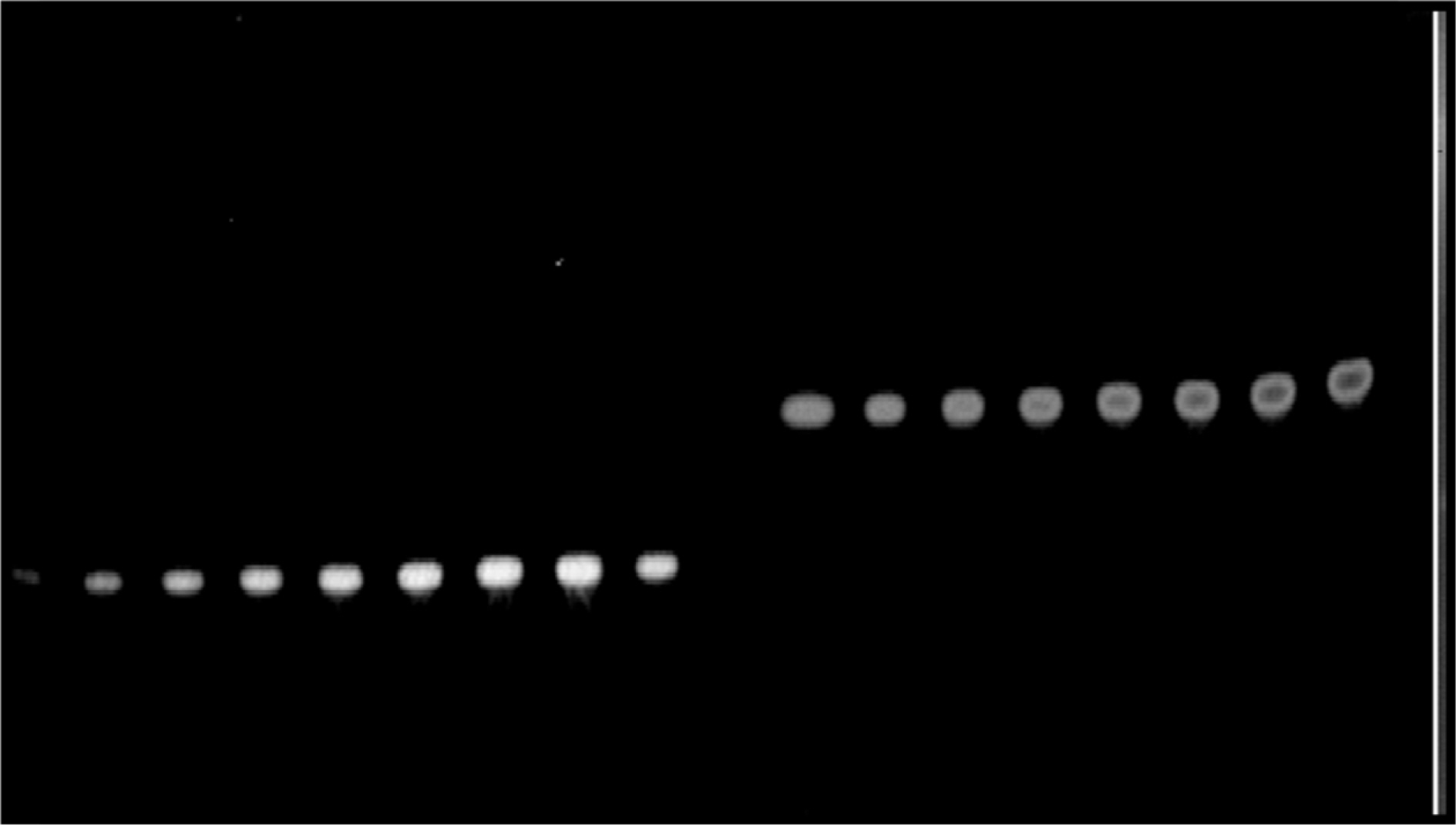
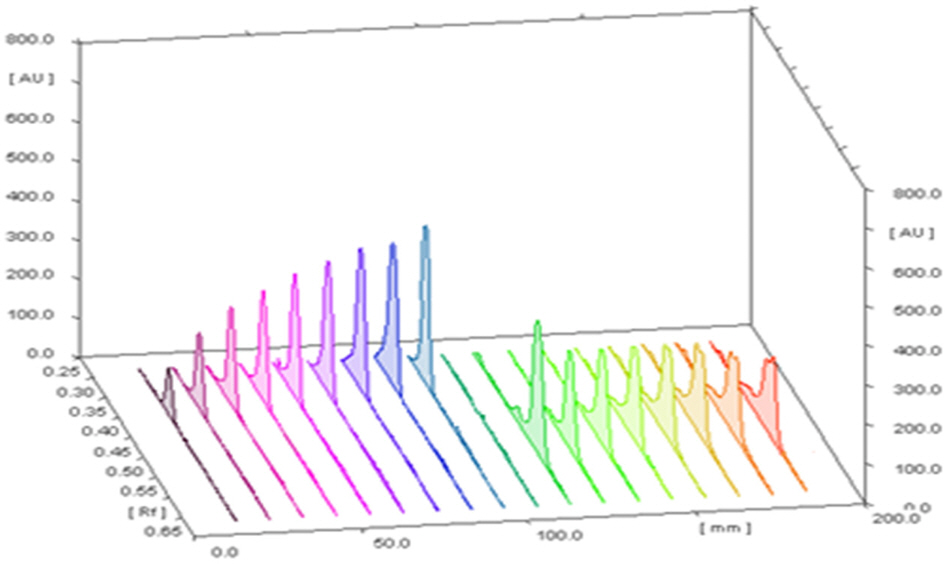
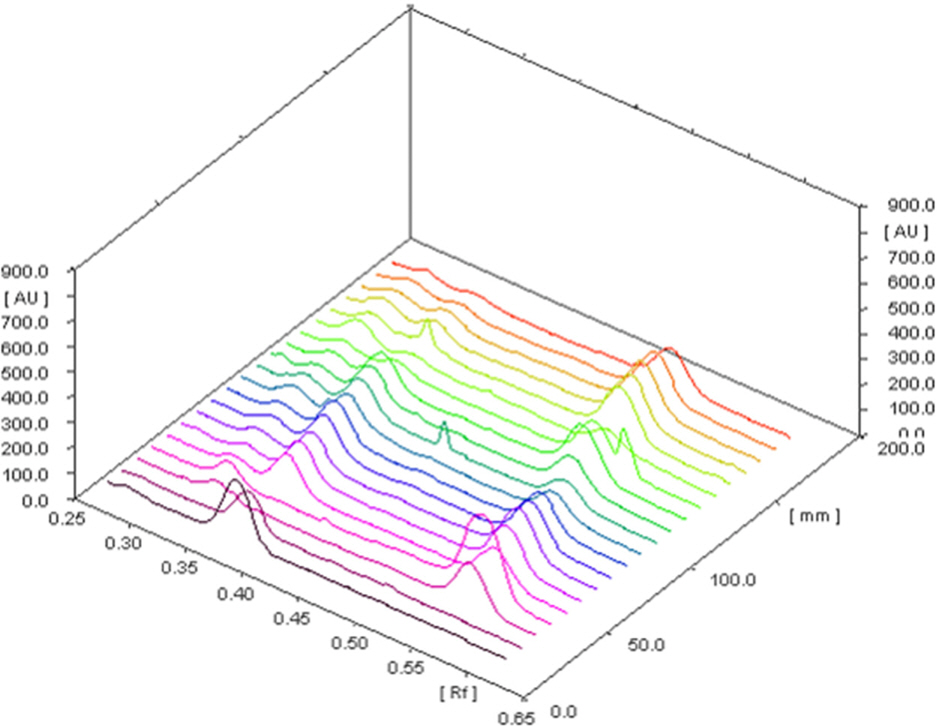
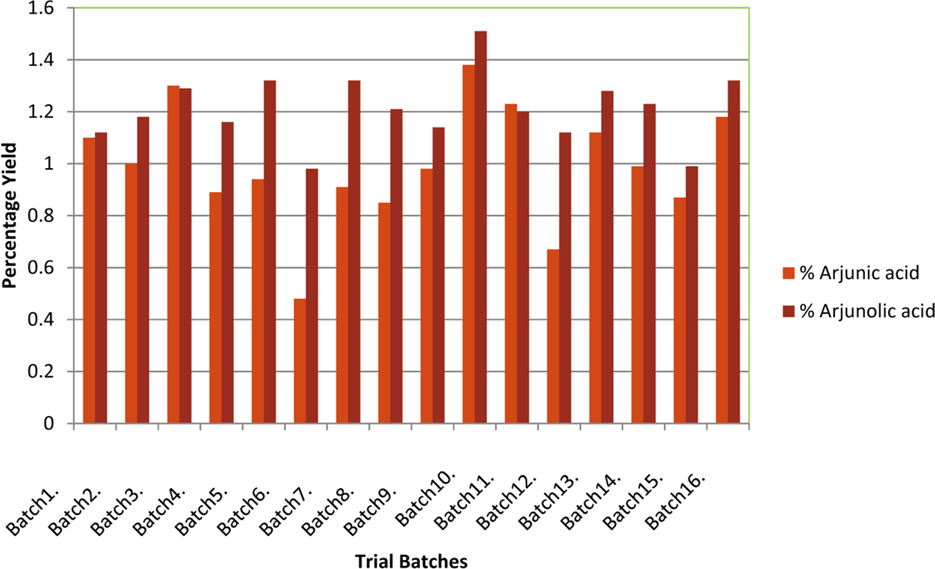
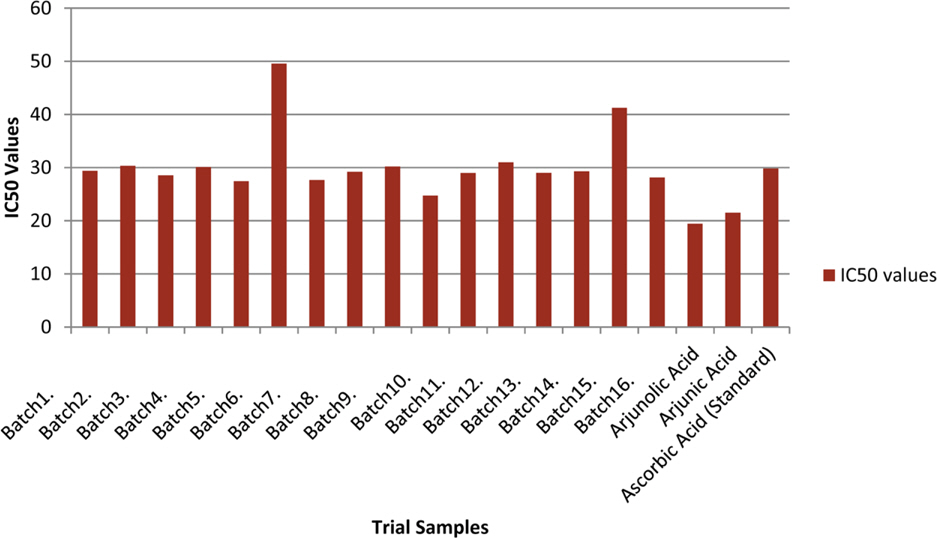
- The optimization and microwave assisted extraction of stem bark of Terminalia arjuna, quantitative estimation of the marker compounds arjunic acid and arjunolic acid using HPTLC and the evaluation of free radical scavenging activity has been performed in this study. The central composite design was used for optimization and the values of parameters for optimized batch of microwave assisted extraction were 1000W (Power), 3 minutes (Time) and 1/120 (Solid/solvent ratio). The solvent system to carry out the HPTLC was toluene: acetic acid: ethyl acetate (5: 5: 0.5) and quantitative estimation was done using standard equations obtained from the marker compounds. The in-vitro free radical scavenging activity was performed spectrophotometrically using ascorbic acid as standard. The value of estimated percentage yield of arjunic acid and arjunolic acid was 1.42% and 1.52% which upon experimentation was obtained as 1.38% and 1.51% respectively. The DPPH assay of the different batches of microwave assisted extraction and marker compounds taken suggested that the marker compounds arjunic acid and the arjunolic acid were responsible for the free radical scavenging activity as the batch having the maximum percentage yield of the marker compounds showed best free radical scavenging effect as compared to standard ascorbic acid. The ICâ‚…â‚€ value of the optimized batch was found to be 24.72 while that of the standard ascorbic acid was 29.83. Hence, the yield of arjunic acid and arjunolic acid has direct correlation with the free radical scavenging activity of stem bark extract of Terminalia arjuna and have potential to serve as active lead compounds for free radical scavenging activity.
Keyword
Figure
Reference
-
References
(1). Sasidharan S.., Chen Y.., Saravanan D.., Sundram K. M.., Latha L. Y.Afr. J. Tradit. Complement Altern. Med. 2011. 8:1–4.(2). Rufino M. S. M.., Alves R. E.., de Brito E. S.., Pérez-Jiménez J.., Saura-Calixto F.., Mancini-Filho J.Food Chem. 2010. 121:996–1002.(3). Bandaranayake W. M.Wetl. Ecol. Manag. 2002. 10:421–452.
Article(4). Vlachojannis J.., Magora F.., Chrubasik S.Phytother. Res. 2011. 25:1102–1104.(5). Staba E. J.., Chung A. C.Phytochemistry. 1981. 20:2495–2498.(6). Aslam J.., Mujib A.., Nasim S. A.., Sharma M. P.Sci. Hortic. 2009. 119:325–329.(7). Xie P.., Chen S.., Liang Y. Z.., Wang X.., Tian R.., Upton R. J.Chromatogr. A. 2006. 1112:171–180.(8). Pozharitskaya O. N.., Ivanova S. A.., Shikov A. N.., Makarov V. G. J.Sep. Sci. 2007. 30:1250–1254.(9). Pirisi F. M.., Cabras P.., Cao C. F.., Migliorini M.., Muggelli M. J.Agric. Food Chem. 2000. 48:1191–1196.(10). de la Torre-Carbot K.., Jauregui O.., Gimeno E.., Castellote A. I.., Lamuela-Raventós R. M.., López-Sabater M. C. J.Agric. Food Chem. 2005. 53:4331–4340.(11). Daferera D. J.., Ziogas B. N.., Polissiou M. G. J.Agric. Food Chem. 2000. 48:2576–2581.(12). Akowuah G. A.., Zhari I.., Norhayati I.., Mariam A. J.Food Compos. Anal. 2006. 19:118–126.(13). Mallavadhani U. V.., Sahu G.., Muralidhar J.Pharm. Biol. 2002. 40:508–511.(14). Khatoon S.., Srivastava M.., Rawat A.., Mehrotra S.JPC-J. Planar Chromatogr. Mod. TLC. 2005. 18:364–367.(15). Kumar A.., Lakshman K.., Jayaveera K. N.., Tripathi S. M.., Satish K. V.Jordan J. Pharm. Sci. 2010. 3:63–68.(16). Pereira C. A.., Yariwake J. H.., Lanças F. M.., Wauters J. N.., Tits M.., Angenot L.Phytochem. Anal. 2004. 15:241–248.(17). Mehta M.., Kaur N.., Bhutani K. K.Phytochem. Anal. 2001. 12:91–95.
Article(18). Hawthorne S. B.., Grabanski C. B.., Martin E.., Miller D. J. J.Chromatogr. A. 2000. 892:421–433.(19). De Castro M. D. L.., Garcýa-Ayuso L. E.Anal. Chim. Acta. 1998. 369:1–10.(20). Huddleston J. G.., Willauer H. D.., Swatloski R. P.., Visser A. E.., Rogers R. D.Chem. Commun. 1998. 16:1765–1766.(21). Khan M. K.., Abert-Vian M.., Fabiano-Tixier A. S.., Dangles O.., Chemat F.Food Chem. 2010. 119:851–858.(22). Dai J.., Mumper R. J.Molecules. 2010. 15:7313–7352.(23). Núñez Sellés A. J.., Vélez Castro H. T.., Agüero-Agüero J.., González-González J.., Naddeo F.., De Simone F.., Rastrelli L. J.Agric. Food Chem. 2002. 50:762–766.(24). Pourmortazavi S. M.., Hajimirsadeghi S. S. J.Chromatogr. A. 2007. 1163:2–24.(25). Routray W.., Orsat V.Food Bioprocess Technol. 2012. 5:409–424.(26). Kaufmann B.., Christen P.Phytochem. Anal. 2002. 13:105–113.(27). Lu Y.., Ma W.., Hu R.., Dai X.., Pan Y. J.Chromatogr. A. 2008. 1208:42–46.(28). Spigno G.., De Faveri D. M. J.Food Eng. 2009. 93:210–217.(29). Devgan M.., Nanda A.., Ansari S. H.Pak. J. Pharm. Sci. 2013. 26:973–976.(30). Devgun M.., Nanda A. R. U. N.., Ansari S. H.Acta Pol. Pharm. 2012. 69:475–485.(31). Devgun M.., Nanda A.., Ansari, S. H; Swamy S. K.Res. J. Pharm. Technol. 2010. 3:644–649.(32). Valko M.., Jomova K.., Rhodes C. J.., Kuèa K.., Musílek K.Arch. Toxicol. 2016. 90:1–37.(33). Przyklenk K.., Kloner R. A.Circ. Res. 1989. 64:86–96.(34). Prasad K.., Kalra J.Am. Heart J. 1993. 125:958–973.(35). Puthur J. T.South Indian J. Biol. Sci. 2016. 2:14–17.(36). Galano A.., Mazzone G.., Alvarez-Diduk R.., Marino T.., Alvarez-Idaboy J. R.., Russo N.Annu. Rev. Food Sci. Technol. 2016. 7:335–352.(37). Fang Y.., Luo Y.., Yu P. J.Renew. Sust. Energ. 2016. 8:033103.(38). Choi S.., Kim J. M.., Shin G. H.., Jung T. D.., Oh J. W.., Choi S. H.., Cho B. Y.., Lee J. H.., Lee O. H.FASEB J. 2016. 30:1174–1175.(39). Martinez-Valverde I.., Perago M. J.., Provan G.., Chesson A. J.Sci. Food Agr. 2002. 82:323–330.(40). Duthie G. G.., Duthie S. J.., Kyle J. A.Nut. Res. Rev. 2000. 13:79–106.(41). Halder S.., Bharal N.., Mediratta P. K.., Kaur I.., Sharma K. K.Indian. J. Exp. Biol. 2009. 47:577–583.(42). Chaudhari, M. Mengi S.Phytother. Res.,. 2006. 20:799–805.
Article(43). Chatha S. A. S.., Hussain A. I.., Asad R.., Majeed M.., Aslam N. J.Food Process Technol. 2014. 5:298.(44). Bachaya H. A.., Iqbal Z.., Khan M. N.., Jabbar A.., Gilani A. H.., Din I. U.Int. J. Agric. Biol. 2009. 11:273–278.(45). Sultana B.., Anwar F.., Przybylski R.Food Chem. 2007. 104:1106–1114.(46). Manna P.., Sinha M.., Sil P. C.Arch. Toxicol. 2008. 82:137–149.(47). Elsherbiny N. M.., Eladl M. A.., Al-Gayyar M. M. H.Cytokine. 2016. 77:26–34.(48). Sumitra M.., Manikandan P.., Kumar D. A.., Arutselvan N.., Balakrishna K.., Manohar B. M.., Puvanakrishnan R.Mol. Cell. Biochem. 2001. 224:135–142.
Article(49). Sun F. Y.., Chen X. P.., Wang J. H.., Qin H. L.., Yang S. R.., Du G. H.Am. J. Chin. Med. 2008. 36:197–207.(50). Pharmacopoeia of India. Herbs and Herbal Monographs: Indian Pharmacopoeial Commission; Ministry of Health and Family Welfare: India,. 2010. 8–9.(51). Bondet V.., Brand-Williams W.., Berset C.LWT-Food Sci. Tech. 1997. 30:609–615.(52). Novilla A.., Nawawi A.., Sugihartina G.., Widowati W.Cukurova Med. J. 2014. 39:224–233.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimization of the Extraction Process for Bioactive Compounds from the Root Barks of Moringa oleifera
- Optimization of Extraction Conditions and Quantitative Analysis of Isoquercitrin and Caffeic Acid from Aster scaber
- In vitro antioxidant properties of a ginseng intestinal metabolite IH-901
- Polyphenols in peanut shells and their antioxidant activity: optimal extraction conditions and the evaluation of antiobesity effects
- Antioxidative Activity of Lichen Thamnolia vermicularis in vitro