J Korean Med Sci.
2017 Sep;32(9):1431-1439. 10.3346/jkms.2017.32.9.1431.
Changes in the Serotype Distribution among Antibiotic Resistant Carriage Streptococcus pneumoniae Isolates in Children after the Introduction of the Extended-Valency Pneumococcal Conjugate Vaccine
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea. hoanlee@snu.ac.kr
- 2Department of Pediatrics, Seoul National University Children's Hospital, Seoul, Korea.
- KMID: 2385948
- DOI: http://doi.org/10.3346/jkms.2017.32.9.1431
Abstract
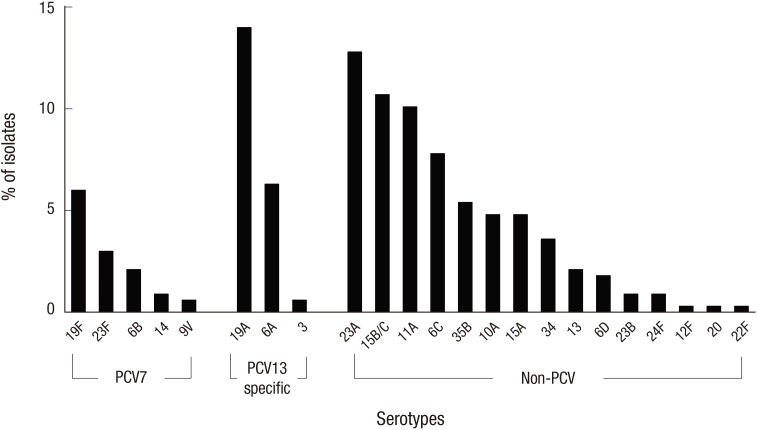
- This study investigated the serotype distribution and antimicrobial resistance of 3,820 nasopharyngeal Streptococcus pneumoniae isolates from infants and children who presented with respiratory symptoms at Seoul National University Children's Hospital from July 2010 to June 2015 after the introduction of the extended-valency pneumococcal conjugate vaccines (PCVs). Serotypes and antimicrobial susceptibility were determined using the Quellung reaction and E-test, respectively. S. pneumoniae was isolated from 397 (10.4%) specimens. The most common serotypes were 19A (14.0%), 23A (12.8%), 15B/C (10.7%), 11A (10.1%), 6C (7.8%), and 6A (6.3%) among the typeable pneumococci (n = 335). The PCV serotype proportions significantly decreased (59.1% in 2010/11 to 17.0% in 2014/15, P < 0.001), whereas the non-PCV serotype proportions significantly increased (40.9% in 2010/11 to 83.0% in 2014/15, P < 0.001). The non-susceptibility rates for penicillin (oral), penicillin (parenteral, non-meningitis), cefotaxime, and erythromycin were 97.8%, 22.8%, 27.7%, and 95.5%, respectively. The proportions of PCV serotypes responsible for non-susceptibility to penicillin (parenteral, non-meningitis) and multidrug resistance significantly decreased (80.8% to 21.1%, P < 0.001 and 64.3% to 12.3%, P < 0.001, respectively), whereas the non-PCV serotype proportions significantly increased (19.2% to 78.9%, P < 0.001 and 35.7% to 87.7%, P < 0.001, respectively). Serotypes 23A and 15B/C demonstrated significant proportional increase among the antibiotics resistant strains. Thus, the PCV serotype proportions decreased and the non-PCV serotype proportions increased among nasopharyngeal carriage pneumococci after the introduction of extended-valency PCVs in Korea. Antimicrobial non-susceptibility rates for penicillin and erythromycin remain high despite the decrease in the proportion of PCV serotypes responsible for antimicrobial resistance over time.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Changes in Serotype of Streptococcus pneumoniae After the Introduction of the 13-Valent Pneumococcal Vaccine in a Homogenous Population on Jeju Island
Jeong Rae Yoo, Sang Taek Heo, Hyunjoo Oh, Suhyun Oh, Young Ree Kim, Keun Hwa Lee
Infect Chemother. 2019;51(1):67-72. doi: 10.3947/ic.2019.51.1.67.
Reference
-
1. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010; 59:1–18.2. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004; 4:144–154. PMID: 14998500.3. Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008; 57:144–148. PMID: 18272956.4. Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011; 11:760–768. PMID: 21621466.5. Hampton LM, Farley MM, Schaffner W, Thomas A, Reingold A, Harrison LH, Lynfield R, Bennett NM, Petit S, Gershman K, et al. Prevention of antibiotic-nonsusceptible Streptococcus pneumoniae with conjugate vaccines. J Infect Dis. 2012; 205:401–411. PMID: 22158567.6. Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015; 15:301–309. PMID: 25656600.7. Gounder PP, Bruce MG, Bruden DJ, Singleton RJ, Rudolph K, Hurlburt DA, Hennessy TW, Wenger J. Effect of the 13-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae--Alaska, 2008–2012. J Infect Dis. 2014; 209:1251–1258. PMID: 24273178.8. Lee GM, Kleinman K, Pelton SI, Hanage W, Huang SS, Lakoma M, Dutta-Linn M, Croucher NJ, Stevenson A, Finkelstein JA. Impact of 13-valent pneumococcal conjugate vaccination on Streptococcus pneumoniae carriage in young children in Massachusetts. J Pediatric Infect Dis Soc. 2014; 3:23–32. PMID: 24567842.9. Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005; 116:e408–e413. PMID: 16140686.10. Millar EV, O'Brien KL, Watt JP, Bronsdon MA, Dallas J, Whitney CG, Reid R, Santosham M. Effect of community-wide conjugate pneumococcal vaccine use in infancy on nasopharyngeal carriage through 3 years of age: a cross-sectional study in a high-risk population. Clin Infect Dis. 2006; 43:8–15. PMID: 16758412.11. Park SY, Moore MR, Bruden DL, Hyde TB, Reasonover AL, Harker-Jones M, Rudolph KM, Hurlburt DA, Parks DJ, Parkinson AJ, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J. 2008; 27:335–340. PMID: 18316986.12. Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009; 124:e1–e11. PMID: 19564254.13. Desai AP, Sharma D, Crispell EK, Baughman W, Thomas S, Tunali A, Sherwood L, Zmitrovich A, Jerris R, Satola SW, et al. Decline in pneumococcal nasopharyngeal carriage of vaccine serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in children in Atlanta, Georgia. Pediatr Infect Dis J. 2015; 34:1168–1174. PMID: 26226445.14. Richter SS, Diekema DJ, Heilmann KP, Dohrn CL, Riahi F, Doern GV. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob Agents Chemother. 2014; 58:6484–6489. PMID: 25136018.15. Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013; 28:1283–1288. PMID: 24015031.16. Yang HI, Park EY, Kim MY. National immunization survey in South Korea, 2013. Public Health Wkly Rep. 2013; 7:449–454.17. Choi EH, Lee HJ, Cho EY, Oh CE, Eun BW, Lee J, Kim MJ. Prevalence and genetic structures of Streptococcus pneumoniae serotype 6D, South Korea. Emerg Infect Dis. 2010; 16:1751–1753. PMID: 21029535.18. Meats E, Brueggemann AB, Enright MC, Sleeman K, Griffiths DT, Crook DW, Spratt BG. Stability of serotypes during nasopharyngeal carriage of Streptococcus pneumoniae . J Clin Microbiol. 2003; 41:386–392. PMID: 12517877.19. Clinical and Laboratory Standards Institute (US). Performance Standards for Antimicrobial Susceptibility testing: Twenty-Fourth Informational Supplement (CLSI Document M100-S24). Wayne, PA: National Committee for Clinical and Laboratory Standards;2014.20. Cho EY, Kang HM, Lee J, Kang JH, Choi EH, Lee HJ. Changes in serotype distribution and antibiotic resistance of nasopharyngeal isolates of Streptococcus pneumoniae from children in Korea, after optional use of the 7-valent conjugate vaccine. J Korean Med Sci. 2012; 27:716–722. PMID: 22787364.21. Moore MR, Hyde TB, Hennessy TW, Parks DJ, Reasonover AL, Harker-Jones M, Gove J, Bruden DL, Rudolph K, Parkinson A, et al. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J Infect Dis. 2004; 190:2031–2038. PMID: 15529269.22. Dunais B, Bruno-Bazureault P, Carsenti-Dellamonica H, Touboul P, Pradier C. A decade-long surveillance of nasopharyngeal colonisation with Streptococcus pneumoniae among children attending day-care centres in south-eastern France: 1999–2008. Eur J Clin Microbiol Infect Dis. 2011; 30:837–843. PMID: 21611871.23. Choe YJ, Lee HJ, Lee H, Oh CE, Cho EY, Choi JH, Kang HM, Yoon IA, Jung HJ, Choi EH. Emergence of antibiotic-resistant non-vaccine serotype pneumococci in nasopharyngeal carriage in children after the use of extended-valency pneumococcal conjugate vaccines in Korea. Vaccine. 2016; 34:4771–4776. PMID: 27546875.24. Kaur R, Casey JR, Pichichero ME. Emerging Streptococcus pneumoniae strains colonizing the nasopharynx in children after 13-valent pneumococcal conjugate vaccination in comparison to the 7-valent era, 2006–2015. Pediatr Infect Dis J. 2016; 35:901–906. PMID: 27420806.25. Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J. 2012; 31:297–301. PMID: 22330166.26. van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MP, Harrison TG, Ladhani SN, Miller E. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine. 2014; 32(9):4349–4355. PMID: 24657717.27. Mameli C, Fabiano V, Daprai L, Bedogni G, Faccini M, Garlaschi ML, Penagini F, Dilillo D, Torresani E, Gramegna M, et al. A longitudinal study of Streptococcus pneumoniae carriage in healthy children in the 13-valent pneumococcal conjugate vaccine era. Hum Vaccin Immunother. 2015; 11:811–817. PMID: 25751237.28. Bryant KA, Block SL, Baker SA, Gruber WC, Scott DA; PCV13 Infant Study Group. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010; 125:866–875. PMID: 20435707.29. Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013; 57:952–962. PMID: 23804191.30. Grant LR, O’Brien SE, Burbidge P, Haston M, Zancolli M, Cowell L, Johnson M, Weatherholtz RC, Reid R, Santosham M, et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS One. 2013; 8:e74906. PMID: 24086394.31. Nahm MH, Lin J, Finkelstein JA, Pelton SI. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis. 2009; 199:320–325. PMID: 19099489.32. Dagan R, Juergens C, Trammel J, Patterson S, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible Streptococcus pneumoniae . J Infect Dis. 2015; 211:1144–1153. PMID: 25355940.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trends in Serotype Distribution of Clinical Isolates of Streptococcus pneumoniae: A Single Center Experience from 2001 to 2006
- Changes in Serotype Distribution and Antibiotic Resistance of Nasopharyngeal Isolates of Streptococcus pneumoniae from Children in Korea, after Optional Use of the 7-Valent Conjugate Vaccine
- Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines
- Serotype Distribution of Pneumococcus Isolated from the Ear Discharge in Children with Otitis Media in 2001–2006
- Direct and Indirect Effects of Pneumococcal Protein Conjugate Vaccine