J Bacteriol Virol.
2017 Mar;47(1):1-13. 10.4167/jbv.2017.47.1.1.
Advances in Epidemiology, Biology and Laboratory Diagnosis of Zika Virus
- Affiliations
-
- 1Department of Bio-industrial Technologies, Konkuk University, Seoul, Korea.
- 2Korean Institute of Science and Technology Information, Seoul, Korea. yungoh@kangwon.ac.kr
- KMID: 2384416
- DOI: http://doi.org/10.4167/jbv.2017.47.1.1
Abstract
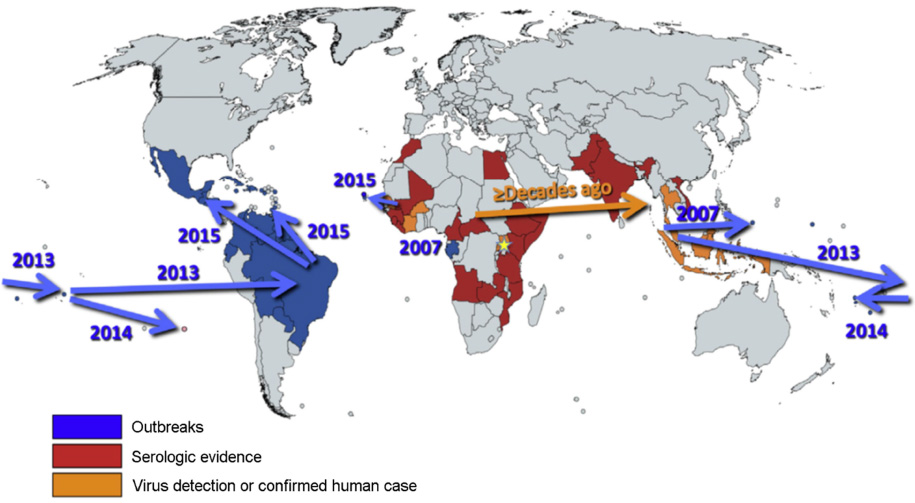
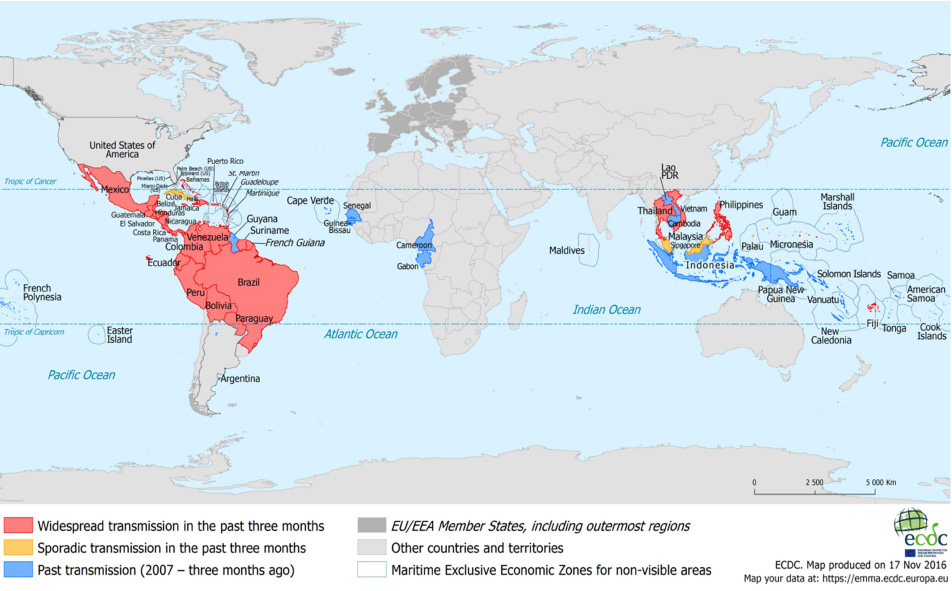
- Zika virus (ZIKV) was spread to both eastward and westward from Uganda where the virus was identified approximately in 1947 by a group of arbovirus researchers. In 2015, ZIKV reached Americas with major outbreaks in Brazil. Most countries with mosquito transmitted ZIKV infection are located in tropical and subtropical areas, where ZIKV is endemic with other flaviviruses, including JEV, dengue and yellow fever virus. Approximately 40 countries in Central and South Americas and territories in South Pacific Islands and South East Asia show autochthonous ZIKV endemics. American lineage of ZIKV is known significantly to be mutated in susceptibility to host and in pathogenicity from Asian and Asian lineages approximately since 2014. Early and specific identification of ZIKV infection is very important for the effective management of patients. First of all, optimal collection of specimens for the laboratory diagnosis is required for both nucleic acid testing (NAT) and serological tests. Specimens for NAT tests and serological tests should be determined by the available laboratory resources, work-flow in each laboratory and the geographic areas of specimen collected in addition to days after showing symptoms. Testing strategy for specific differentiation among flaviviruses will vary depending on the prevalence of viruses known to be circulating in the area where the patients were exposed. NAT will be employed for the patients presenting with onset of symptoms less than 7 days. Advanced diagnostic technologies should be continuously developed for the increase of specificity and sensitivity of ZIKV diagnosis.
MeSH Terms
Figure
Reference
-
1. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952; 46:509–520.
Article2. Wikan N, Smith DR. Zika virus: history of a newly emerging arbovirus. Lancet Infect Dis. 2016; 16:e119–e126.
Article3. Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008; 14:1232–1239.
Article4. Chang C, Ortiz K, Ansari A, Gershwin ME. The Zika outbreak of the 21st century. J Autoimmun. 2016; 68:1–13.
Article5. Oduyebo T, Igbinosa I, Petersen EE, Polen KN, Pillai SK, Ailes EC, et al. Update: Interim Guidance for Health Care Providers Caring for Pregnant Women with Possible Zika Virus Exposure - United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016; 65:739–744.
Article6. Epidemiological Update: Neurological syndrome, congenital anomalies and Zika virus infection. Washington, D.C.: PAHO/WHO;2016.7. Jouannic JM, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia. Lancet. 2016; 387:1051–1052.
Article8. Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009; 15:1347–1350.
Article9. Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016; 387:719–721.
Article10. Dichtelmüller HO, Biesert L, Fabbrizzi F, Gajardo R, Gröner A, von Hoegen I, et al. Robustness of solvent/detergent treatment of plasma derivatives: a data collection from Plasma Protein Therapeutics Association member companies. Transfusion. 2009; 49:1931–1943.
Article11. Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954; 48:139–145.
Article12. Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969; 18:411–415.13. Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, et al. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 2016; 130:69–80.
Article14. Gardner LM, Chen N, Sarkar S. Global risk of Zika virus depends critically on vector status of Aedes albopictus. Lancet Infect Dis. 2016; 16:522–523.
Article15. Rapid risk assessment: Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome. Stockholm: ECDC;2015.16. Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus. Lancet Infect Dis. 2016; 16:523.
Article17. Kim K, Shresta S. Neuroteratogenic Viruses and Lessons for Zika Virus Models. Trends Microbiol. 2016; 24:622–636.
Article18. Park Y, Noh Y, Ryu B, Shin SH, Ra S. Preparedness plan and response to Zika virus infection in the Republic of Korea. Public Health Wkly Rep. 2016; 9:426–429.19. Ramos da Silva S, Gao SJ. Zika virus: An update on epidemiology, pathology, molecular biology, and animal model. J Med Virol. 2016; 88:1291–1296.
Article20. Dupont-Rouzeyrol M, Biron A, O'Connor O, Huguon E, Descloux E. Infectious Zika viral particles in breastmilk. Lancet. 2016; 387:1051.
Article21. Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016; 16:405.
Article22. Kaddumukasa MA, Mutebi JP, Lutwama JJ, Masembe C, Akol AM. Mosquitoes of Zika Forest, Uganda: species composition and relative abundance. J Med Entomol. 2014; 51:104–113.
Article23. Terzian AC, Mondini A, Bronzoni RV, Drumond BP, Ferro BP, Cabrera EM, et al. Detection of Saint Louis encephalitis virus in dengue-suspected cases during a dengue 3 outbreak. Vector Borne Zoonotic Dis. 2011; 11:291–300.
Article24. Ayres CF. Identification of Zika virus vectors and implications for control. Lancet Infect Dis. 2016; 16:278–279.
Article25. Kramer LD. Complexity of virus-vector interactions. Curr Opin Virol. 2016; 21:81–86.
Article26. World Health Organization on Regulations for Transport of Infectious Substances. 2015-2016. Geneva: WHO;2015.27. Gubler D, Kuno G, Markoff L. Flaviviruses. In : Fields BN, Knipe DM, Howley PM, editors. Fields virology. 5th edition. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins;2007.28. Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, et al. The 3.8 A resolution cryo-EM structure of Zika virus. Science. 2016; 352:467–470.29. Gadea G, Bos S, Krejbich-Trotot P, Clain E, Viranaicken W, El-Kalamouni C, et al. A robust method for the rapid generation of recombinant Zika virus expressing the GFP reporter gene. Virology. 2016; 497:157–162.
Article30. Giovanetti M, Faria NR, Nunes MR, de Vasconcelos JM, Lourenco J, Rodrigues SG, et al. Zika virus complete genome from Salvador, Bahia, Brazil. Infect Genet Evol. 2016; 41:142–145.
Article31. Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol. 2015; 68:53–55.
Article32. Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016; 16:1000–1001.
Article33. Faye O, Dupressoir A, Weidmann M, Ndiaye M, Alpha Sall A. One-step RT-PCR for detection of Zika virus. J Clin Virol. 2008; 43:96–101.
Article34. Campos Rde M, Cirne-Santos C, Meira GL, Santos LL, de Meneses MD, Friedrich J, et al. Prolonged detection of Zika virus RNA in urine samples during the ongoing Zika virus epidemic in Brazil. J Clin Virol. 2016; 77:69–70.
Article35. Carocci M, Yang PL. Lactimidomycin is a broad-spectrum inhibitor of dengue and other RNA viruses. Antiviral Res. 2016; 128:57–62.
Article36. Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, et al. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 2010; 6:209–217.
Article37. Micoine K, Persich P, Llaveria J, Lam MH, Maderna A, Loganzo F, et al. Total syntheses and biological reassessment of lactimidomycin, isomigrastatin and congener glutarimide antibiotics. Chemistry. 2013; 19:7370–7383.
Article38. Garreau de Loubresse N, Prokhorova I, Holtkamp W, Rodnina MV, Yusupova G, Yusupov M. Structural basis for the inhibition of the eukaryotic ribosome. Nature. 2014; 513:517–522.
Article39. Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Res. 2002; 30:1292–1305.
Article40. Niemz A, Ferguson TM, Boyle DS. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011; 29:240–250.
Article41. Fernanda Estofolete C, Terzian AC, Parreira R, Esteves A, Hardman L, Greque GV, et al. Clinical and laboratory profile of Zika virus infection in dengue suspected patients: A case series. J Clin Virol. 2016; 81:25–30.
Article42. Moulin E, Selby K, Cherpillod P, Kaiser L, Boillat-Blanco N. Simultaneous outbreaks of dengue, chikungunya and Zika virus infections: diagnosis challenge in a returning traveller with nonspecific febrile illness. New Microbes New Infect. 2016; 11:6–7.
Article43. Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A. 2016; 113:7852–7857.
Article44. Calvo EP, Sánchez-Quete F, Durán S, Sandoval I, Castellanos JE. Easy and inexpensive molecular detection of dengue, chikungunya and zika viruses in febrile patients. Acta Trop. 2016; 163:32–37.
Article45. Ehrmeyer SS, Laessig RH. Point-of-care testing, medical error, and patient safety: a 2007 assessment. Clin Chem Lab Med. 2007; 45:766–773.
Article46. World Health Organization. Laboratory biosafety manual. 3rd edition. Geneva: WHO;2004.47. Chambers TJ, Monath TP. The Flaviviruses: Detection, Diagnosis and Vaccine Development. 1st edition. Academic Press;2003.48. World Health Organization. Laboratory testing for Zika virus infection (Interim guidance). Geneva: WHO;2016.49. World Health Organization. Consultation on the documentary evidence and independent performance evaluation requirement for the emergency use assessment and listing procedure for Zika virus in vitro diagnostics. Geneva: WHO;2016.50. CDC. Guidance for U.S. Laboratories Testing for Zika Virus Infection. 2016.51. Rabe IB, Staples JE, Villanueva J, Hummel KB, Johnson JA, Rose L, et al. Interim Guidance for Interpretation of Zika Virus Antibody Test Results. MMWR Morb Mortal Wkly Rep. 2016; 65:543–546.
Article52. Tian B, Qiu Z, Ma J, Zardán Gómez de la Torre T, Johansson C, Svedlindh P, et al. Attomolar Zika virus oligonucleotide detection based on loop-mediated isothermal amplification and AC susceptometry. Biosens Bioelectron. 2016; 86:420–425.
Article53. Xu MY, Liu SQ, Deng CL, Zhang QY, Zhang B. Detection of Zika virus by SYBR green one-step real-time RT-PCR. J Virol Methods. 2016; 236:93–97.
Article54. Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell. 2016; 165:1255–1266.
Article