Endocrinol Metab.
2017 Jun;32(2):265-273. 10.3803/EnM.2017.32.2.265.
Predetermined Anti-Diabetic Drug Regimen Adjustments during Ramadan Fasting: An Observational Study of Safety
- Affiliations
-
- 1Department of Internal Medicine, The University of Jordan School of Medicine, Amman, Jordan.
- 2Department of Internal Medicine, King Hussein Cancer Center (KHCC), Amman, Jordan.
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Jordan University Hospital, The University of Jordan School of Medicine, Amman, Jordan. baraaayman@gmail.com
- 4The University of Jordan Faculty of Pharmacy, Amman, Jordan.
- KMID: 2384086
- DOI: http://doi.org/10.3803/EnM.2017.32.2.265
Abstract
- BACKGROUND
Many Muslim type 2 diabetes mellitus (T2DM) patients choose to fast the month of Ramadan despite the possible adverse health effects brought about by the change in dietary habits, among other things. Clinical data regarding the safety of multi-drug regimens during fasting are particularly scarce. The aim of the study was to evaluate the safety of a drug protocol devised by the authors to accommodate Ramadan's dietary changes, involving dose adjustments of four anti-diabetic drug regimens in T2DM patients fasting Ramadan.
METHODS
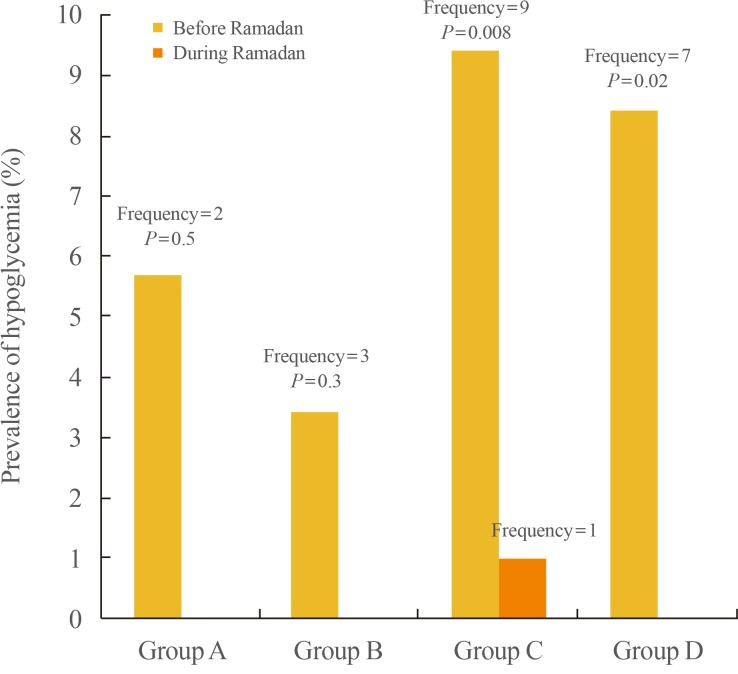
In this prospective, observational, open-label study, 301 T2DM patients who wished to fast Ramadan were followed during Ramadan and the preceding month. The incidence of hypoglycemia, diabetic ketoacidosis (DKA) and non-ketotic hyperosmolar state (NKHS) was monitored. Patients were classified into four groups: A group (those taking metformin, sulfonylurea and insulin [n=33]); B group (metformin and sulfonylurea [n=89]); C group (metformin and insulin [n=96]); and D group (premixed 70/30, glargine or regular insulin [n=82]). During Ramadan, drug doses were adjusted as percentages of their pre-Ramadan values: 75% for sulfonylureas, 75% for glargine, 75% for premixed insulin 70/30 in two doses, and 75% for regular insulin. Metformin was adjusted to a twice-daily regimen.
RESULTS
No cases of DKA or NKHS were reported. Hypoglycemia occurred at a lower rate than pre-Ramadan values in groups C, and D; and a similar rate in groups A, and B.
CONCLUSION
The data suggested that using the above protocol to adjust the doses of anti-diabetic drugs is safe in T2DM patients in regards to hypoglycemia, DKA, and NKHS.
Keyword
MeSH Terms
Figure
Reference
-
1. Al-Arouj M, Bouguerra R, Buse J, Hafez S, Hassanein M, Ibrahim MA, et al. Recommendations for management of diabetes during Ramadan. Diabetes Care. 2005; 28:2305–2311. PMID: 16123509.
Article2. Salti I, Benard E, Detournay B, Bianchi-Biscay M, Le Brigand C, Voinet C, et al. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. 2004; 27:2306–2311. PMID: 15451892.3. Hassan A, Meo SA, Usmani AM, Shaikh TJ. Diabetes during Ramadan: pre-approach model: presentation, risk stratification, education. Eur Rev Med Pharmacol Sci. 2014; 18:1798–1805. PMID: 24992624.4. Al-Arouj M, Assaad-Khalil S, Buse J, Fahdil I, Fahmy M, Hafez S, et al. Recommendations for management of diabetes during Ramadan: update 2010. Diabetes Care. 2010; 33:1895–1902. PMID: 20668157.5. Karamat MA, Syed A, Hanif W. Review of diabetes management and guidelines during Ramadan. J R Soc Med. 2010; 103:139–147. PMID: 20382905.6. Akbani MF, Saleem M, Gadit WU, Ahmed M, Basit A, Malik RA. Fasting and feasting safely during Ramadan in the patient with diabetes. Prac Diabetes Int. 2005; 22:100–104.
Article7. Pathan MF, Sahay RK, Zargar AH, Raza SA, Khan AK, Ganie MA, et al. South Asian consensus guideline: use of insulin in diabetes during Ramadan. Indian J Endocrinol Metab. 2012; 16:499–502. PMID: 22837903.
Article8. Almaatouq MA. Pharmacological approaches to the management of type 2 diabetes in fasting adults during Ramadan. Diabetes Metab Syndr Obes. 2012; 5:109–119. PMID: 22654520.
Article9. Zargar AH, Siraj M, Jawa AA, Hasan M, Mahtab H. Maintenance of glycaemic control with the evening administration of a long acting sulphonylurea in male type 2 diabetic patients undertaking the Ramadan fast. Int J Clin Pract. 2010; 64:1090–1094. PMID: 20455956.
Article10. Salti I. Diabetes and Ramadan Study Group. Efficacy and safety of insulin glargine and glimepiride in subjects with type 2 diabetes before, during and after the period of fasting in Ramadan. Diabet Med. 2009; 26:1255–1261. PMID: 20002478.
Article11. Hui E, Bravis V, Salih S, Hassanein M, Devendra D. Comparison of Humalog Mix 50 with human insulin Mix 30 in type 2 diabetes patients during Ramadan. Int J Clin Pract. 2010; 64:1095–1099. PMID: 20337752.
Article12. Devendra D, Gohel B, Bravis V, Hui E, Salih S, Mehar S, et al. Vildagliptin therapy and hypoglycaemia in Muslim type 2 diabetes patients during Ramadan. Int J Clin Pract. 2009; 63:1446–1450. PMID: 19678856.
Article13. Al Sifri S, Basiounny A, Echtay A, Al Omari M, Harman-Boehm I, Kaddaha G, et al. The incidence of hypoglycaemia in Muslim patients with type 2 diabetes treated with sitagliptin or a sulphonylurea during Ramadan: a randomised trial. Int J Clin Pract. 2011; 65:1132–1140. PMID: 21951832.
Article14. Cesur M, Corapcioglu D, Gursoy A, Gonen S, Ozduman M, Emral R, et al. A comparison of glycemic effects of glimepiride, repaglinide, and insulin glargine in type 2 diabetes mellitus during Ramadan fasting. Diabetes Res Clin Pract. 2007; 75:141–147. PMID: 16815586.
Article15. Workgroup on Hypoglycemia, American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005; 28:1245–1249. PMID: 15855602.16. Boon NA, Davidson S, Britton R. Davidson's principles & practice of medicine. 20th ed. Edinburgh: Elsevier/Churchill Livingstone;2008.17. Mafauzy M. Repaglinide versus glibenclamide treatment of type 2 diabetes during Ramadan fasting. Diabetes Res Clin Pract. 2002; 58:45–53. PMID: 12161056.
Article18. Mafauzy M, Mohammed WB, Anum MY, Zulkifli A, Ruhani AH. A study of the fasting diabetic patients during the month of Ramadan. Med J Malaysia. 1990; 45:14–17. PMID: 2152063.19. Akram J, De Verga V. Insulin lispro (Lys(B28), Pro(B29) in the treatment of diabetes during the fasting month of Ramadan. Ramadan Study Group. Diabet Med. 1999; 16:861–866. PMID: 10547214.20. Ahmed MH, Abdu TA. Diabetes and Ramadan: an update on use of glycemic therapies during fasting. Ann Saudi Med. 2011; 31:402–406. PMID: 21727749.
Article21. Hassanein M, Hanif W, Malik W, Kamal A, Geransar P, Lister N, et al. Comparison of the dipeptidyl peptidase-4 inhibitor vildagliptin and the sulphonylurea gliclazide in combination with metformin, in Muslim patients with type 2 diabetes mellitus fasting during Ramadan: results of the VECTOR study. Curr Med Res Opin. 2011; 27:1367–1374. PMID: 21568833.
Article22. Malik S, Lopez V, Chen R, Wu W, Wong ND. Undertreatment of cardiovascular risk factors among persons with diabetes in the United States. Diabetes Res Clin Pract. 2007; 77:126–133. PMID: 17118478.
Article23. Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002; 287:360–372. PMID: 11790216.24. Al-Arouj M, Hassoun AA, Medlej R, Pathan MF, Shaltout I, Chawla MS, et al. The effect of vildagliptin relative to sulphonylureas in Muslim patients with type 2 diabetes fasting during Ramadan: the VIRTUE study. Int J Clin Pract. 2013; 67:957–963. PMID: 24001317.25. Anwar A, Azmi KN, Hamidon BB, Khalid BA. An open label comparative study of glimepiride versus repaglinide in type 2 diabetes mellitus Muslim subjects during the month of Ramadan. Med J Malaysia. 2006; 61:28–35. PMID: 16708731.26. Aravind SR, Al Tayeb K, Ismail SB, Shehadeh N, Kaddaha G, Liu R, et al. Hypoglycaemia in sulphonylurea-treated subjects with type 2 diabetes undergoing Ramadan fasting: a five-country observational study. Curr Med Res Opin. 2011; 27:1237–1242. PMID: 21506631.
Article27. Aravind SR, Ismail SB, Balamurugan R, Gupta JB, Wadhwa T, Loh SM, et al. Hypoglycemia in patients with type 2 diabetes from India and Malaysia treated with sitagliptin or a sulfonylurea during Ramadan: a randomized, pragmatic study. Curr Med Res Opin. 2012; 28:1289–1296. PMID: 22738801.
Article28. Hassanein M, Abdallah K, Schweizer A. A double-blind, randomized trial, including frequent patient-physician contacts and Ramadan-focused advice, assessing vildagliptin and gliclazide in patients with type 2 diabetes fasting during Ramadan: the STEADFAST study. Vasc Health Risk Manag. 2014; 10:319–326. PMID: 24920915.29. Bravis V, Hui E, Salih S, Mehar S, Hassanein M, Devendra D. Ramadan Education and Awareness in Diabetes (READ) programme for Muslims with type 2 diabetes who fast during Ramadan. Diabet Med. 2010; 27:327–331. PMID: 20536496.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ramadan fasting following laparoscopic sleeve gastrectomy: a prospective online survey cohort study in Egypt
- Effect of a Diabetic Camp Program on the Fasting Blood Sugar Level in Type 2 Diabetic Patients
- The Perioperative Management of Diabetic Patients: A Retrospective Study
- The Association Between Fasting C-peptide and Gastrointestinal Symptoms of Gastroparesis in Type 2 Diabetic Patients
- CORRECTION: Ramadan fasting following laparoscopic sleeve gastrectomy: a prospective online survey cohort study in Egypt