J Periodontal Implant Sci.
2017 Jun;47(3):182-192. 10.5051/jpis.2017.47.3.182.
Characteristics of contact and distance osteogenesis around modified implant surfaces in rabbit tibiae
- Affiliations
-
- 1Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. pros53@snu.ac.kr
- 2Department of Prosthodontics, Seoul National University School of Dentistry, Seoul, Korea.
- KMID: 2383832
- DOI: http://doi.org/10.5051/jpis.2017.47.3.182
Abstract
- PURPOSE
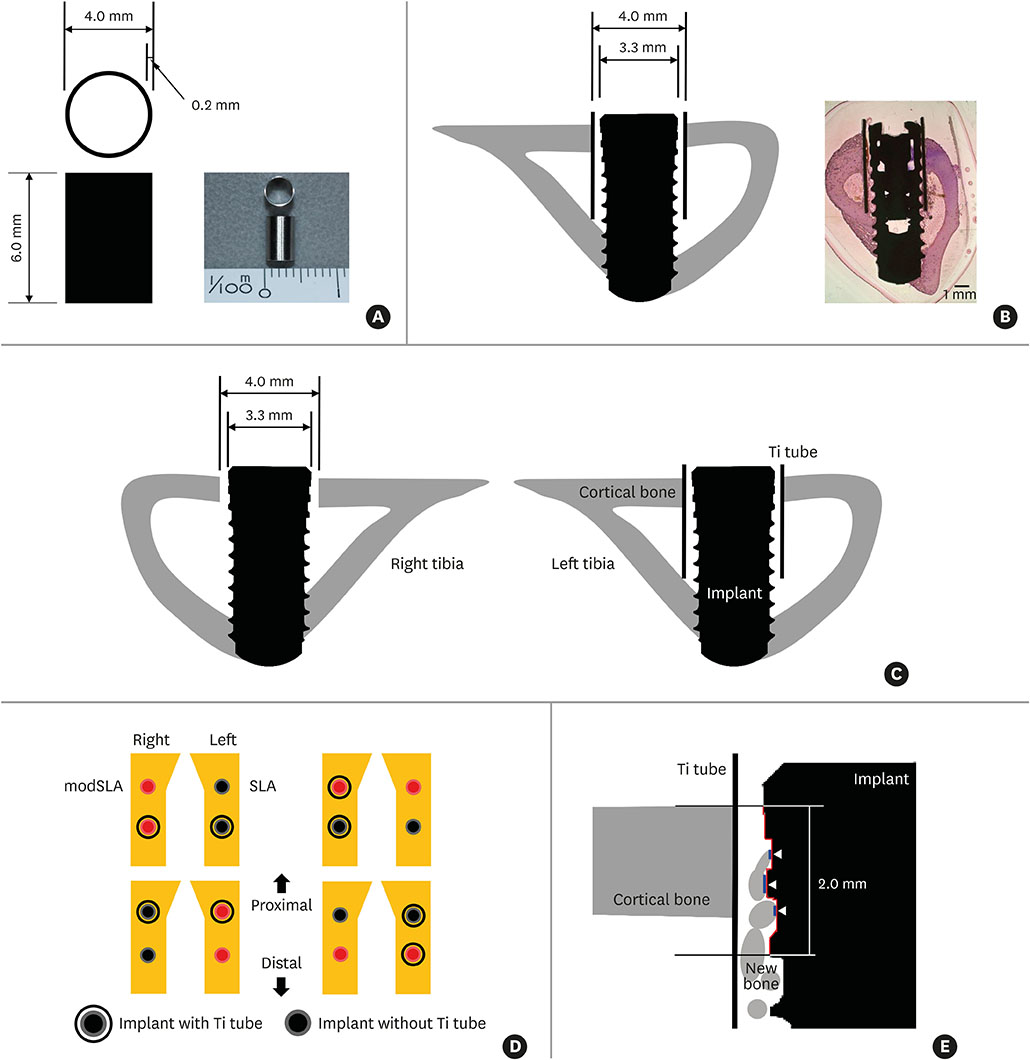
Contact and distance osteogenesis occur around all endosseous dental implants. However, the mechanisms underlying these processes have not been fully elucidated. We hypothesized that these processes occur independently of each other. To test this, we used titanium (Ti) tubes to physically separate contact and distance osteogenesis, thus allowing contact osteogenesis to be measured in the absence of possible triggers from distance osteogenesis.
METHODS
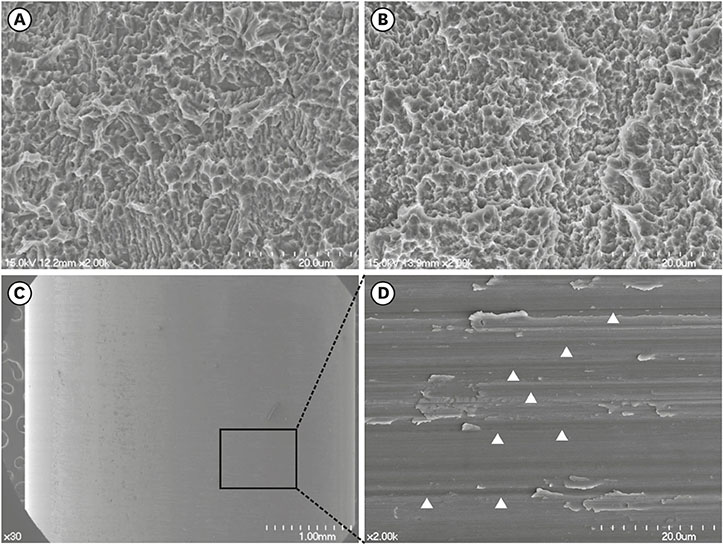
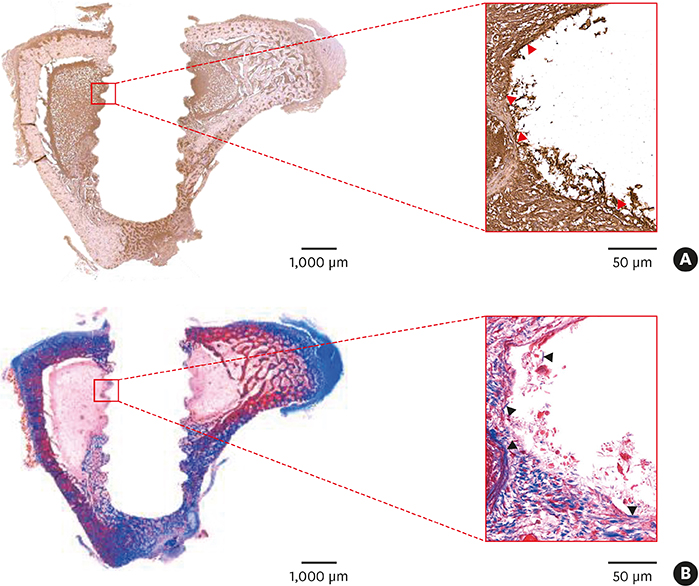
Sandblasted and acid-etched (SLA) and modified SLA (modSLA) implants were used. Both types had been sandblasted with large grit and then etched with acid. The modSLA implants then underwent additional treatment to increase hydrophilicity. The implants were implanted into rabbit tibiae, and half were implanted within Ti tubes. The bone-to-implant contact (BIC) ratio was calculated for each implant. Immunohistochemical analyses of bone morphogenetic protein (BMP)-2 expression and new bone formation (Masson trichrome stain) were performed.
RESULTS
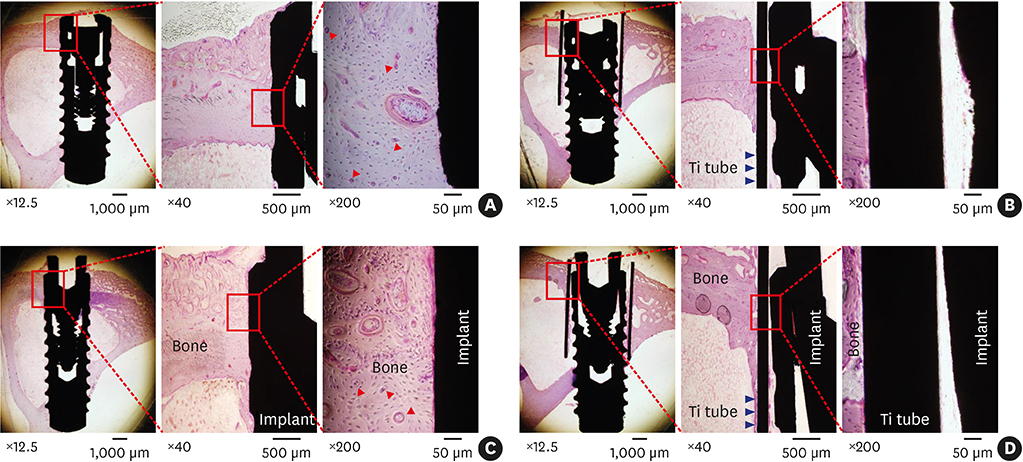
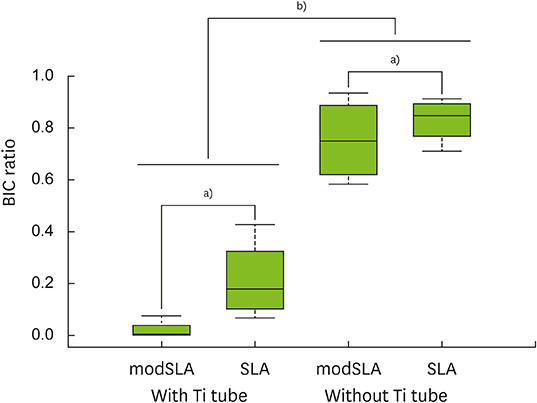
The implants outside of Ti tubes were associated with good bone formation along the implant surface. Implantation within a Ti tube significantly reduced the BIC ratio (P<0.001). Compared with the modSLA implants, the SLA implants were associated with significantly higher BIC ratios, regardless of the presence or absence of Ti tubes (P=0.043). In the absence of Ti tubes, the bone adjacent to the implant had areas of new bone formation that expressed BMP-2 at high levels.
CONCLUSIONS
This study disproved the null hypothesis and suggested that contact osteogenesis is initiated by signals from the old bone that undergoes distance osteogenesis after drilling. This signal may be BMP-2.
Keyword
MeSH Terms
Figure
Reference
-
1. Osborn J, Newesely H. Dynamic aspects of the implant-bone interface. In : Heimke G, editor. Dental implants. Münche: Verlag;1980. p. 111–123.2. Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003; 14:251–262.3. Davies JE. In vitro modeling of the bone/implant interface. Anat Rec. 1996; 245:426–445.4. Moreo P, García-Aznar JM, Doblaré M. Bone ingrowth on the surface of endosseous implants. Part 1: mathematical model. J Theor Biol. 2009; 260:1–12.
Article5. Sela MN, Badihi L, Rosen G, Steinberg D, Kohavi D. Adsorption of human plasma proteins to modified titanium surfaces. Clin Oral Implants Res. 2007; 18:630–638.
Article6. Terheyden H, Lang NP, Bierbaum S, Stadlinger B. Osseointegration--communication of cells. Clin Oral Implants Res. 2012; 23:1127–1135.7. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage. 2012; 20:256–260.
Article8. Yeo IS, Han JS, Yang JH. Biomechanical and histomorphometric study of dental implants with different surface characteristics. J Biomed Mater Res B Appl Biomater. 2008; 87:303–311.
Article9. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982; 11:318–326.
Article10. Berglundh T, Lindhe J, Jonsson K, Ericsson I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol. 1994; 21:189–193.
Article11. Nygren H, Tengvall P, Lundström I. The initial reactions of TiO2 with blood. J Biomed Mater Res. 1997; 34:487–492.
Article12. Park JY, Davies JE. Red blood cell and platelet interactions with titanium implant surfaces. Clin Oral Implants Res. 2000; 11:530–539.
Article13. Ammann AJ, Beck LS, DeGuzman L, Hirabayashi SE, Lee WP, McFatridge L, et al. Transforming growth factor-beta. Effect on soft tissue repair. Ann N Y Acad Sci. 1990; 593:124–134.14. Brighton CT. The biology of fracture repair. Instr Course Lect. 1984; 33:60–82.15. Brunski JB. In vivo bone response to biomechanical loading at the bone/dental-implant interface. Adv Dent Res. 1999; 13:99–119.
Article16. Cromack DT, Pierce GF, Mustoe TA. TGF-beta and PDGF mediated tissue repair: identifying mechanisms of action using impaired and normal models of wound healing. Prog Clin Biol Res. 1991; 365:359–373.17. Ikeda K, Takeshita S. Factors and mechanisms involved in the coupling from bone resorption to formation: how osteoclasts talk to osteoblasts. J Bone Metab. 2014; 21:163–167.
Article18. Mustoe TA, Pierce GF, Morishima C, Deuel TF. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991; 87:694–703.
Article19. Pfeilschifter J, Wolf O, Naumann A, Minne HW, Mundy GR, Ziegler R. Chemotactic response of osteoblastlike cells to transforming growth factor beta. J Bone Miner Res. 1990; 5:825–830.
Article20. Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987; 165:251–256.
Article21. Deuel TF, Senior RM, Huang JS, Griffin GL. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982; 69:1046–1049.
Article22. Huang JS, Huang SS, Kennedy B, Deuel TF. Platelet-derived growth factor. Specific binding to target cells. J Biol Chem. 1982; 257:8130–8136.
Article23. Park JY, Gemmell CH, Davies JE. Platelet interactions with titanium: modulation of platelet activity by surface topography. Biomaterials. 2001; 22:2671–2682.
Article24. Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, et al. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989; 109:429–440.
Article25. Seppä H, Grotendorst G, Seppä S, Schiffmann E, Martin GR. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982; 92:584–588.
Article26. Urist MR. Bone: formation by autoinduction. Science. 1965; 150:893–899.
Article27. El Bialy I, Jiskoot W, Reza Nejadnik M. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm Res. 2017; 34:1152–1170.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stability and Efficacy of Titanium in Osteointegration in a Rabbit Model
- Effect of implant surface microtopography by hydroxyapatite grit-blasting on adhesion, proliferation, and differentiation of osteoblast-like cell line, MG-63
- Histomorphometric evaluation of osteogenesis with brushite implant surfaces in dogs
- Comparison between TiUniteTM and another oxidized implant using the rabbit tibia model
- Influence of the adjacent periodontium and inter-implant distance on bone resorption around non-submerged implants: A retrospective clinical and radiographic study