Ann Rehabil Med.
2017 Feb;41(1):25-33. 10.5535/arm.2017.41.1.25.
Hemodynamic Adaptations to Regular Exercise in People With Spinal Cord Injury
- Affiliations
-
- 1Department of Rehabilitation Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. eyeini@naver.com
- 2Center for Exercise Medicine, Yonsei University, Wonju, Korea.
- 3Department of Physiology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2383674
- DOI: http://doi.org/10.5535/arm.2017.41.1.25
Abstract
OBJECTIVE
To investigate the real-time cardiovascular response to the progressive overload exercise in different levels of spinal cord injury (SCI), and to find out whether regular exercise has effect on these cardiovascular responses.
METHODS
The study enrolled 8 able-bodied individuals in the control group plus 15 SCI subjects who were divided into two groups by their neurological level of injury: high-level SCI group (T6 or above) and low-level SCI group (T7 or below). Also, subjects were divided into exercise group and non-exercise group by usual exercise habits. We instructed the subjects to perform exercises using arm ergometer according to the protocol and checked plethysmograph for the real time assessment of blood pressure, heart rate, and cardiac output.
RESULTS
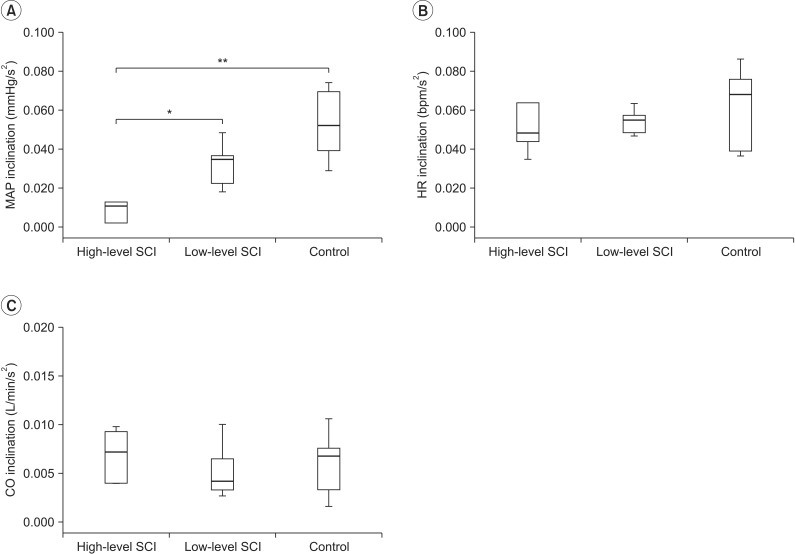
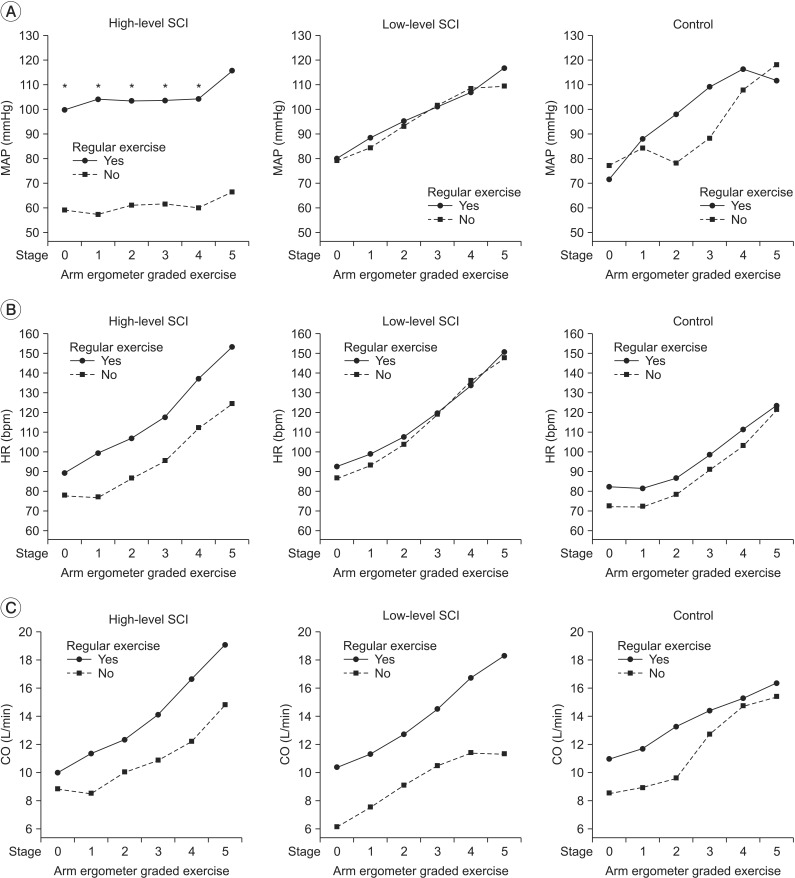
Six subjects were included in high-level SCI group (3 cervical, 3 thoracic injuries), 9 subjects in low-level SCI group (9 thoracic injuries), and 8 able-bodied individuals in control group. During arm ergometer-graded exercise, mean arterial pressure (MAP) was significantly lower in high-level SCI subjects of non-exercise group, compared with high-level SCI subjects of exercise group. In addition, HR was significantly higher in low-level SCI group compared with control group.
CONCLUSION
There are significant differences in mean arterial pressure of high-level SCI group according to usual exercise habits. We discovered that even in non-athlete high-level SCI, regular exercise can bring cardiac modulation through blood pressure control.
Keyword
MeSH Terms
Figure
Reference
-
1. Clausen JP. Circulatory adjustments to dynamic exercise and effect of physical training in normal subjects and in patients with coronary artery disease. Prog Cardiovasc Dis. 1976; 18:459–495. PMID: 6992.
Article2. Garstang SV, Miller-Smith SA. Autonomic nervous system dysfunction after spinal cord injury. Phys Med Rehabil Clin N Am. 2007; 18:275–296. PMID: 17543773.
Article3. Gondim FA, Lopes AC Jr, Oliveira GR, Rodrigues CL, Leal PR, Santos AA, et al. Cardiovascular control after spinal cord injury. Curr Vasc Pharmacol. 2004; 2:71–79. PMID: 15320835.
Article4. Krassioukov A, Claydon VE. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res. 2006; 152:223–229. PMID: 16198703.
Article5. Bravo G, Guízar-Sahagun G, Ibarra A, Centurion D, Villalon CM. Cardiovascular alterations after spinal cord injury: an overview. Curr Med Chem Cardiovasc Hematol Agents. 2004; 2:133–148. PMID: 15320796.
Article6. Krassioukov A. Which pathways must be spared in the injured human spinal cord to retain cardiovascular control? Prog Brain Res. 2006; 152:39–47. PMID: 16198692.
Article7. Krassioukov AV, Bunge RP, Pucket WR, Bygrave MA. The changes in human spinal sympathetic preganglionic neurons after spinal cord injury. Spinal Cord. 1999; 37:6–13. PMID: 10025688.
Article8. Krassioukov AV, Weaver LC. Reflex and morphological changes in spinal preganglionic neurons after cord injury in rats. Clin Exp Hypertens. 1995; 17:361–373. PMID: 7735281.
Article9. Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma. 2006; 23:1713–1725. PMID: 17184183.
Article10. Claydon VE, Hol AT, Eng JJ, Krassioukov AV. Cardiovascular responses and postexercise hypotension after arm cycling exercise in subjects with spinal cord injury. Arch Phys Med Rehabil. 2006; 87:1106–1114. PMID: 16876557.
Article11. Hopman MT, Oeseburg B, Binkhorst RA. Cardiovascular responses in persons with paraplegia to prolonged arm exercise and thermal stress. Med Sci Sports Exerc. 1993; 25:577–583. PMID: 8492685.
Article12. Rimaud D, Calmels P, Roche F, Mongold JJ, Trudeau F, Devillard X. Effects of graduated compression stockings on cardiovascular and metabolic responses to exercise and exercise recovery in persons with spinal cord injury. Arch Phys Med Rehabil. 2007; 88:703–709. PMID: 17532890.
Article13. Campbell IG, Williams C, Lakomy HK. Physiological and metabolic responses of wheelchair athletes in different racing classes to prolonged exercise. J Sports Sci. 2004; 22:449–456. PMID: 15160598.
Article14. Barfield JP, Malone LA, Collins JM, Ruble SB. Disability type influences heart rate response during power wheelchair sport. Med Sci Sports Exerc. 2005; 37:718–723. PMID: 15870623.
Article15. Dixon EM, Kamath MV, McCartney N, Fallen EL. Neural regulation of heart rate variability in endurance athletes and sedentary controls. Cardiovasc Res. 1992; 26:713–719. PMID: 1423437.
Article16. Yamamoto K, Miyachi M, Saitoh T, Yoshioka A, Onodera S. Effects of endurance training on resting and post-exercise cardiac autonomic control. Med Sci Sports Exerc. 2001; 33:1496–1502. PMID: 11528338.
Article17. Otsuka Y, Shima N, Moritani T, Okuda K, Yabe K. Orthostatic influence on heart rate and blood pressure variability in trained persons with tetraplegia. Eur J Appl Physiol. 2008; 104:75–78. PMID: 18542988.
Article18. Wecht JM, Marsico R, Weir JP, Spungen AM, Bauman WA, De Meersman RE. Autonomic recovery from peak arm exercise in fit and unfit individuals with paraplegia. Med Sci Sports Exerc. 2006; 38:1223–1228. PMID: 16826018.
Article19. Schmid A, Huonker M, Stober P, Barturen JM, Schmidt-Trucksass A, Durr H, et al. Physical performance and cardiovascular and metabolic adaptation of elite female wheelchair basketball players in wheelchair ergometry and in competition. Am J Phys Med Rehabil. 1998; 77:527–533. PMID: 9862541.20. Asayama K, Nakamura Y, Ogata H, Hatada K, Okuma H, Deguchi Y. Physical fitness of paraplegics in full wheelchair marathon racing. Paraplegia. 1985; 23:277–287. PMID: 4069738.
Article21. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985; 10:141–146. PMID: 4053261.22. Gappmaier E. The Submaximal Clinical Exercise Tolerance Test (SXTT) to establish safe exercise prescription parameters for patients with chronic disease and disability. Cardiopulm Phys Ther J. 2012; 23:19–29.23. Gass GC, Watson J, Camp EM, Court HJ, McPherson LM, Redhead P. The effects of physical training on high level spinal lesion patients. Scand J Rehabil Med. 1980; 12:61–65. PMID: 7209438.24. Mathias CJ, Frankel HL. Autonomic disturbances in spinal cord lesions. In : Mathias CJ, Bannister R, editors. Autonomic failure: a textbook of clinical disorders of the autonomic nervous system. 4th ed. Oxford: Oxford University Press;2002. p. 494–513.25. Claydon VE, Elliott SL, Sheel AW, Krassioukov A. Cardiovascular responses to vibrostimulation for sperm retrieval in men with spinal cord injury. J Spinal Cord Med. 2006; 29:207–216. PMID: 16859224.
Article26. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000; 81:506–516. PMID: 10768544.
Article27. Currie KD, West CR, Hubli M, Gee CM, Krassioukov AV. Peak heart rates and sympathetic function in tetraplegic nonathletes and athletes. Med Sci Sports Exerc. 2015; 47:1259–1264. PMID: 25211366.
Article28. Schmid A, Huonker M, Barturen JM, Stahl F, Schmidt-Trucksass A, Konig D, et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol (1985). 1998; 85:635–641. PMID: 9688742.
Article29. Dela F, Mohr T, Jensen CM, Haahr HL, Secher NH, Biering-Sorensen F, et al. Cardiovascular control during exercise: insights from spinal cord-injured humans. Circulation. 2003; 107:2127–2133. PMID: 12695298.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Regular Exercise on Cardiopulmonary Fitness in Males With Spinal Cord Injury
- Delayed Spinal Cord Injury Following Low Voltage Electrical Accident
- The Effect of Midodrine on Exercise-induced Hypotension in Cervical Cord Injury Patients
- Spinal Cord Injury Followign Electrical Accidents : Case Reports
- Effects of Electrical Stimulation and Weight-Supported Treadmill Gait Simulation on Apoptosis in the Muscles of Rats with Spinal Cord Injury