J Pathol Transl Med.
2015 Jan;49(1):37-43. 10.4132/jptm.2014.10.25.
Transglutaminase 2 Expression and Its Prognostic Significance in Clear Cell Renal Cell Carcinoma
- Affiliations
-
- 1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. blue7270@snu.ac.kr
- 2Department of Pathology, Good Moonhwa Hospital, Busan, Korea.
- 3Kidney Research Institute, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2381351
- DOI: http://doi.org/10.4132/jptm.2014.10.25
Abstract
- BACKGROUND
A few recent studies have demonstrated a possible role of transglutaminase 2 (TG2) in tumorigenesis or progression of renal cell carcinoma (RCC). The aim of this study was to examine TG2 expression and its clinicopathologic significance in a large number of human clear cell RCCs (CCRCCs).
METHODS
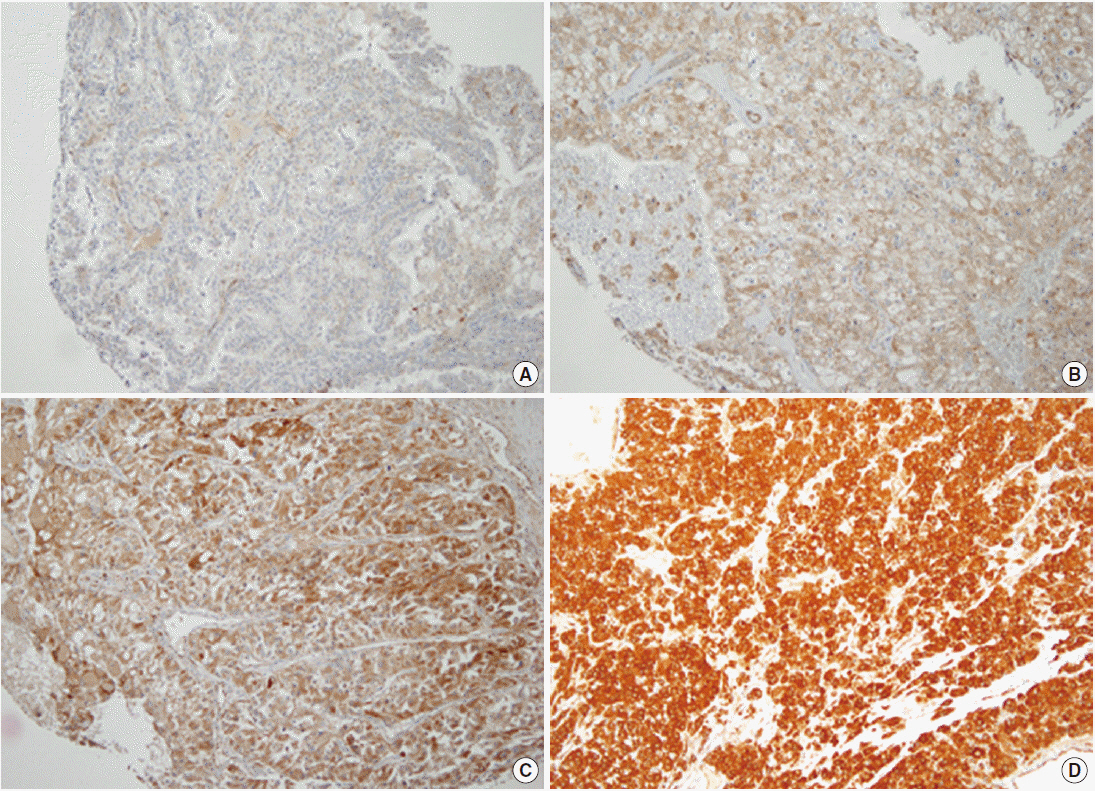
We analyzed 638 CCRCC patients who underwent partial or radical nephrectomy between 1995 and 2005. The expression of TG2 was determined by immunohistochemistry and categorized into four groups, according to staining intensity: negative (0), mild (1+), moderate (2+), and strong (3+).
RESULTS
TG2 staining intensity was negative in 8.5% of CCRCC (n=54), 1+ in 32.6% (n=208), 2+ in 50.5% (n=322), and 3+ in 8.5% (n=54). Strong TG2 expression was correlated with high Fuhrman nuclear grade (p=.011), high T category (p=.049), metastasis (p=.043) and male sex (p<.001) but not with N category.The survival analysis showed a significant association between strong TG2 expression and worse overall and cancer-specific survival (p=.027 and p=.010, respectively). On multivariate analysis, strong TG2 expression was a marginally significant prognostic indicator for Fuhrman nuclear grade and TNM staging (p=.054).
CONCLUSIONS
Our study is the first to demonstrate the clinicopathologic significance of TG2 expression in a large number of human CCRCC samples. Strong TG2 expression was associated with high nuclear grade and poor prognosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011; 60:615–21.
Article2. Ljungberg B, Hanbury DC, Kuczyk MA, et al. Renal cell carcinoma guideline. Eur Urol. 2007; 51:1502–10.
Article3. Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep. 2005; 6:7–18.
Article4. Ljungberg B. Prognostic markers in renal cell carcinoma. Curr Opin Urol. 2007; 17:303–8.
Article5. Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003; 4:140–56.
Article6. Greenberg CS, Birckbichler PJ, Rice RH. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991; 5:3071–7.
Article7. Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2009; 36:659–70.
Article8. Gaudry CA, Verderio E, Aeschlimann D, Cox A, Smith C, Griffin M. Cell surface localization of tissue transglutaminase is dependent on a fibronectin-binding site in its N-terminal beta-sandwich domain. J Biol Chem. 1999; 274:30707–14.9. Gaudry CA, Verderio E, Jones RA, Smith C, Griffin M. Tissue transglutaminase is an important player at the surface of human endothelial cells: evidence for its externalization and its colocalization with the beta(1) integrin. Exp Cell Res. 1999; 252:104–13.10. Belkin AM. Extracellular TG2: emerging functions and regulation. Febs J. 2011; 278:4704–16.
Article11. Wang Z, Griffin M. TG2, a novel extracellular protein with multiple functions. Amino Acids. 2012; 42:939–49.
Article12. Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res. 2004; 10:8068–76.
Article13. Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene. 2007; 26:2459–70.
Article14. Hwang JY, Mangala LS, Fok JY, et al. Clinical and biological significance of tissue transglutaminase in ovarian carcinoma. Cancer Res. 2008; 68:5849–58.
Article15. Verma A, Wang H, Manavathi B, et al. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006; 66:10525–33.
Article16. Verma A, Guha S, Diagaradjane P, et al. Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res. 2008; 14:2476–83.17. Park KS, Kim HK, Lee JH, et al. Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer. J Cancer Res Clin Oncol. 2010; 136:493–502.
Article18. Fok JY, Ekmekcioglu S, Mehta K, et al. Implications of tissue transglutaminase expression in malignant melanoma. Mol Cancer Ther. 2006; 5:1493–503.
Article19. Kumar S, Mehta K. Tissue transglutaminase, inflammation, and cancer: how intimate is the relationship? Amino Acids. 2013; 44:81–8.
Article20. Lentini A, Abbruzzese A, Provenzano B, Tabolacci C, Beninati S. Transglutaminases: key regulators of cancer metastasis. Amino Acids. 2013; 44:25–32.
Article21. Hidaka H, Seki N, Yoshino H, et al. Tumor suppressive microRNA-1285 regulates novel molecular targets: aberrant expression and functional significance in renal cell carcinoma. Oncotarget. 2012; 3:44–57.
Article22. Erdem M, Erdem S, Sanli O, et al. Up-regulation of TGM2 with ITGB1 and SDC4 is important in the development and metastasis of renal cell carcinoma. Urol Oncol. 2014; 32:25.e13–20.
Article23. Wykoff CC, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Identification of novel hypoxia dependent and independent target genes of the von Hippel-Lindau (VHL) tumour suppressor by mRNA differential expression profiling. Oncogene. 2000; 19:6297–305.24. Kim DS, Choi YB, Han BG, et al. Cancer cells promote survival through depletion of the von Hippel-Lindau tumor suppressor by protein crosslinking. Oncogene. 2011; 30:4780–90.
Article25. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.26. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982; 6:655–63.
Article27. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press;2004.28. Ku BM, Kim DS, Kim KH, et al. Transglutaminase 2 inhibition found to induce p53 mediated apoptosis in renal cell carcinoma. FASEB J. 2013; 27:3487–95.
Article29. Herman JF, Mangala LS, Mehta K. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene. 2006; 25:3049–58.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Significance of E-cadherin Expression in Renal Cell Carcinoma
- Correlation of Clinical Stage and Presumptive Prognostic Factors in Renal Cell Carcinoma
- Expression of E-cadherin in Chromophobe Renal Cell Carcinoma and Its Prognostic Implication
- Letter to the editor: Prognostic significance of preoperative and follow-up neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with non-metastatic clear cell renal cell carcinoma
- Differentiation of Chromophobe Renal Cell Carcinoma and Clear Cell Renal Cell Carcinoma by Using Helical CT