Clin Exp Otorhinolaryngol.
2017 Jun;10(2):148-152. 10.21053/ceo.2016.00948.
Vestibular Neuritis With Minimal Canal Paresis: Characteristics and Clinical Implication
- Affiliations
-
- 1Department of Otorhinolaryngology, Inha University School of Medicine, Incheon, Korea. stedman@inha.ac.kr
- KMID: 2380432
- DOI: http://doi.org/10.21053/ceo.2016.00948
Abstract
OBJECTIVES
To analyze the clinical characteristics of vestibular neuritis patients with minimal canal paresis (canal paresis <25%).
METHODS
Patients clinically diagnosed with vestibular neuritis and treated at our institute (n=201) underwent otoneurological examination and vestibular function tests. Patients were categorized in terms of the results of caloric testing (canal paresis<25%, n=58; canal paresis≥25%, n=143). Clinical characteristics and laboratory outcomes were compared between two groups.
RESULTS
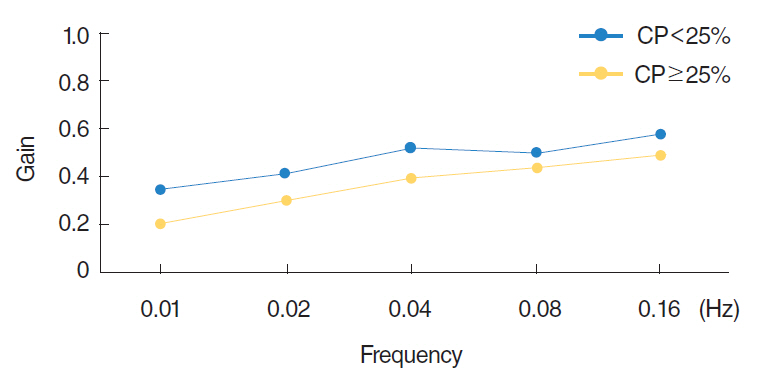
Existence of underlying diseases, preceding symptoms, and direction of spontaneous nystagmus were not different between the groups. The mean duration of spontaneous nystagmus was shortest in the minimal canal paresis group (P<0.001) and the direction of spontaneous nystagmus changed more frequently in this group (P<0.001) during recovery. Among the subgroup with minimal canal paresis, only 29.5% had an abnormal finding on the rotatory chair test, as compared to 81.5% of the canal paresis group. The minimal canal paresis group showed higher sensory organization test scores in computerized dynamic posturography.
CONCLUSION
Patients with minimal canal paresis (canal paresis <25%) show similar clinical manifestations as conventional vestibular neuritis patients, but have faster recovery of symptoms and a higher incidence of recovery nystagmus. This finding support that the minimal canal paresis could be considered as a milder type of vestibular neuritis.
MeSH Terms
Figure
Reference
-
1. Strupp M, Brandt T. Vestibular neuritis. Semin Neurol. 2009; Nov. 29(5):509–19.
Article2. Goddard JC, Fayad JN. Vestibular neuritis. Otolaryngol Clin North Am. 2011; Apr. 44(2):361–5.
Article3. Jeong SH, Kim HJ, Kim JS. Vestibular neuritis. Semin Neurol. 2013; Jul. 33(3):185–94.
Article4. Coats AC. Vestibular neuronitis. Trans Am Acad Ophthalmol Otolaryngol. 1969; May-Jun. 73(3):395–408.5. Anttinen A, Lang AH, Aantaa E, Marttila R. Vestibular neuronitis; a neurological and neurophysiological evaluation. Acta Neurol Scand. 1983; Feb. 67(2):90–6.
Article6. Schuknecht HF, Kitamura K. Second Louis H. Clerf Lecture. Vestibular neuritis. Ann Otol Rhinol Laryngol Suppl. 1981; Jan-Feb. 90(1 Pt 2):1–19.7. Cooper CW. Vestibular neuronitis: a review of a common cause of vertigo in general practice. Br J Gen Pract. 1993; Apr. 43(369):164–7.8. Jongkees LB, Maas JP, Philipszoon AJ. Clinical nystagmography: a detailed study of electro-nystagmography in 341 patients with vertigo. Pract Otorhinolaryngol (Basel). 1962; 24:65–93.9. Arbusow V, Schulz P, Strupp M, Dieterich M, von Reinhardstoettner A, Rauch E, et al. Distribution of herpes simplex virus type 1 in human geniculate and vestibular ganglia: implications for vestibular neuritis. Ann Neurol. 1999; Sep. 46(3):416–9.
Article10. Theil D, Arbusow V, Derfuss T, Strupp M, Pfeiffer M, Mascolo A, et al. Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol. 2001; Oct. 11(4):408–13.
Article11. Cnyrim CD, Newman-Toker D, Karch C, Brandt T, Strupp M. Bedside differentiation of vestibular neuritis from central “vestibular pseudoneuritis”. J Neurol Neurosurg Psychiatry. 2008; Apr. 79(4):458–60.
Article12. Mandala M, Nuti D, Broman AT, Zee DS. Effectiveness of careful bedside examination in assessment, diagnosis, and prognosis of vestibular neuritis. Arch Otolaryngol Head Neck Surg. 2008; Feb. 134(2):164–9.
Article13. Newman-Toker DE, Kattah JC, Alvernia JE, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. 2008; Jun. 70(24 Pt 2):2378–85.
Article14. Baloh RW, Honrubia V, Kerber KA. Baloh and Honrubia’s clinical neurophysiology of the vestibular system. 4th ed. New York (NY): Oxford University Press;2011.15. Choi KD, Oh SY, Kim HJ, Koo JW, Cho BM, Kim JS. Recovery of vestibular imbalances after vestibular neuritis. Laryngoscope. 2007; Jul. 117(7):1307–12.
Article16. Halmagyi GM, Aw ST, Karlberg M, Curthoys IS, Todd MJ. Inferior vestibular neuritis. Ann N Y Acad Sci. 2002; Apr. 956:306–13.
Article17. Kim JS, Kim HJ. Inferior vestibular neuritis. J Neurol. 2012; Aug. 259(8):1553–60.
Article18. Proctor L, Glackin R. Factors contributing to variability of caloric test scores. Acta Otolaryngol. 1985; Sep-Oct. 100(3-4):161–71.
Article19. Baertschi AJ, Johnson RN, Hanna GR. A theoretical and experimental determination of vestibular dynamics in caloric stimulation. Biol Cybern. 1975; Nov. 20(3-4):175–86.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lateralizing Value of Romberg Test and Modified Romberg Test in Acute Unilateral Vestibular Neuritis
- A Case of Pseudo-Vestibular Neuritis with Contralesional Canal Paresis due to Spontaneous Bilateral Vertebral Artery Dissection
- Clinical Implication and Proposed Mechanism of Direction Changing Vibration Induced Nystagmus in Unilateral Vestibular Hypofunction
- Clinical Aspect of Acute Vestibular Neuritis
- Change of Subjective Visual Vertical (SVV) in Patients of Vestibular Neuritis