J Korean Med Sci.
2017 Jul;32(7):1124-1130. 10.3346/jkms.2017.32.7.1124.
Subcutaneous Immunotherapy for Allergic Asthma in a Single Center of Korea: Efficacy, Safety, and Clinical Response Predictors
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. ye9007@ajou.ac.kr
- 2Clinical Trial Center, Ajou University Medical Center, Suwon, Korea.
- KMID: 2379607
- DOI: http://doi.org/10.3346/jkms.2017.32.7.1124
Abstract
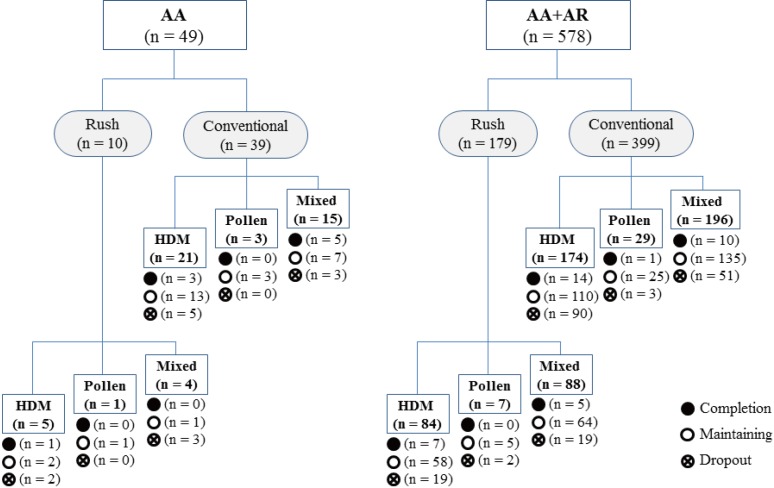
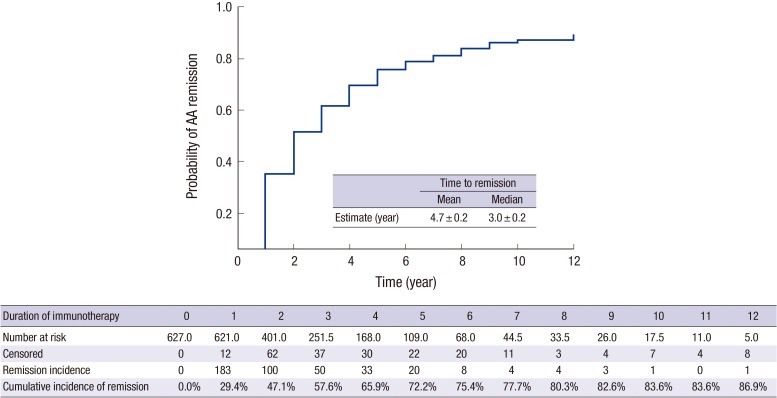
- Allergen-specific immunotherapy is the only causal treatment for allergic diseases. However, the efficacy of immunotherapy may vary around the world due to differences in climate, the nature of aero-allergens and their distribution. The aim of this study was to describe the effects of subcutaneous immunotherapy (SCIT) in Korean adults with allergic asthma (AA). As a retrospective cohort study, we reviewed medical records for 627 patients with AA in Korea who were sensitized to house dust mite (HDM) and/or pollens and who underwent SCIT with aluminum hydroxide adsorbed allergen extract from 2000 to 2012. Rates of remission, defined as no further requirement of maintenance medication, over time were determined by means of life tables and extension of survival analysis. Herein, 627 asthmatic patients achieved remission within a mean of 4.7 ± 0.2 years. The cumulative incidence rates of remission from AA were 86.9% upon treatment with SCIT. Baseline forced expiratory volume in the first second (FEV1) ≥ 80% (hazard ratio [HR], 3.10; 95% confidence interval [CI], 1.79-5.39; P < 0.001), and maintenance of immunotherapy for more than 3 years (HR, 1.82; 95% CI, 1.21-2.72; P = 0.004) were significant predictors of asthma remission during SCIT. In 284 patients on SCIT with HDM alone, initial specific immunoglobulin E (IgE) levels to Dermatophagoides pteronyssinus and Dermatophagoides farinae did not show significant difference between remission and non-remission group after adjusting demographic variables. In conclusion, SCIT was effective and safe treatment modality for patients with AA. Initial FEV1 ≥ 80% and immunotherapy more than 3 years were found to be associated with favorable clinical responses to SCIT.
MeSH Terms
-
Adult
Aluminum Hydroxide
Asthma*
Climate
Cohort Studies
Dermatophagoides farinae
Dermatophagoides pteronyssinus
Forced Expiratory Volume
Humans
Immunoglobulin E
Immunoglobulins
Immunotherapy*
Incidence
Korea*
Life Tables
Medical Records
Pollen
Pyroglyphidae
Retrospective Studies
Aluminum Hydroxide
Immunoglobulin E
Immunoglobulins
Figure
Reference
-
1. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014; 133:621–631. PMID: 24581429.2. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197. PMID: 26922928.3. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010; CD001186. PMID: 20687065.4. Bauer CS, Rank MA. Comparative efficacy and safety of subcutaneous versus sublingual immunotherapy. J Allergy Clin Immunol. 2014; 134:765–765.e2. PMID: 25171871.5. Nelson HS. Subcutaneous immunotherapy versus sublingual immunotherapy: which is more effective? J Allergy Clin Immunol Pract. 2014; 2:144–149. PMID: 24607040.6. Casale TB, Stokes JR. Immunotherapy: what lies beyond. J Allergy Clin Immunol. 2014; 133:612–619. PMID: 24581428.7. Kim ME, Kim JE, Sung JM, Lee JW, Choi GS, Nahm DH. Safety of accelerated schedules of subcutaneous allergen immunotherapy with house dust mite extract in patients with atopic dermatitis. J Korean Med Sci. 2011; 26:1159–1164. PMID: 21935270.8. Rönmark E, Jönsson E, Lundbäck B. Remission of asthma in the middle aged and elderly: report from the Obstructive Lung Disease in Northern Sweden study. Thorax. 1999; 54:611–613. PMID: 10377206.9. Panhuysen CI, Vonk JM, Koëter GH, Schouten JP, van Altena R, Bleecker ER, Postma DS. Adult patients may outgrow their asthma: a 25-year follow-up study. Am J Respir Crit Care Med. 1997; 155:1267–1272. PMID: 9105065.10. Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010; 125:569–574. PMID: 20144472.11. Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013; 132:1322–1336. PMID: 24139829.12. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, Cox L, Demoly P, Frew AJ, O’Hehir R, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015; 136:556–568. PMID: 26162571.13. Tabar AI, Arroabarren E, Echechipía S, García BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. J Allergy Clin Immunol. 2011; 127:57–63. 63.e1–63.e3. PMID: 21211641.14. Rottem M, Egbarya A. Subcutaneous immunotherapy in Northern Israel: efficacy and safety. Isr Med Assoc J. 2014; 16:539–543. PMID: 25351009.15. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, Nelson H, Akdis CA. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3. PMID: 23498595.16. Di Lorenzo G, Mansueto P, Pacor ML, Rizzo M, Castello F, Martinelli N, Ditta V, Lo Bianco C, Leto-Barone MS, D'Alcamo A, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009; 123:1103–1110. 1110.e1–1110.e4. PMID: 19356792.17. Fujimura T, Yonekura S, Horiguchi S, Taniguchi Y, Saito A, Yasueda H, Inamine A, Nakayama T, Takemori T, Taniguchi M, et al. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin Immunol. 2011; 139:65–74. PMID: 21300571.18. Ciprandi G, Silvestri M. Serum specific IgE: a biomarker of response to allergen immunotherapy. J Investig Allergol Clin Immunol. 2014; 24:35–39.19. Tosca M, Silvestri M, Accogli A, Rossi GA, Ciprandi G. Serum-specific IgE and allergen immunotherapy in allergic children. Immunotherapy. 2014; 6:29–33. PMID: 24341881.20. Passalacqua G. The use of single versus multiple antigens in specific allergen immunotherapy for allergic rhinitis: review of the evidence. Curr Opin Allergy Clin Immunol. 2014; 14:20–24. PMID: 24362238.21. Webber CM, Calabria CW. Assessing the safety of subcutaneous immunotherapy dose adjustments. Ann Allergy Asthma Immunol. 2010; 105:369–375. PMID: 21055663.22. Kartal O, Gulec M, Caliskaner Z, Musabak U, Sener O. Safety of subcutaneous immunotherapy with inhalant allergen extracts: a single-center 30-year experience from Turkey. Immunopharmacol Immunotoxicol. 2015; 37:280–286. PMID: 25858053.23. Santos N, Pereira AM, Silva R, Torres da Costa J, Plácido JL. Characterisation of systemic reactions to subcutaneous immunotherapy with airborne allergens and classification according to WAO 2010. Allergol Immunopathol (Madr). 2015; 43:25–31. PMID: 24661594.24. Cox L. Advantages and disadvantages of accelerated immunotherapy schedules. J Allergy Clin Immunol. 2008; 122:432–434. PMID: 18619664.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sublingual immunotherapy for allergic rhinitis
- Allergen-Specific Immunotherapy Against Allergic Respiratory Diseases
- Allergen-specific immunotherapy in pediatric allergic asthma
- Efficacy and Safety of Subcutaneous Allergen Immunotherapy for Allergic Rhinitis
- Update of Sublingual Immunotherapy for Allergic Rhinitis