J Breast Cancer.
2017 Mar;20(1):82-90. 10.4048/jbc.2017.20.1.82.
Cardioprotective Effect of Dexrazoxane in Patients with HER2-Positive Breast Cancer Who Receive Anthracycline Based Adjuvant Chemotherapy Followed by Trastuzumab
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 2Division of Cardiology, Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Division of Breast-Thyroid Surgery, Department of Surgery, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. medics21@catholic.ac.kr
- KMID: 2379402
- DOI: http://doi.org/10.4048/jbc.2017.20.1.82
Abstract
- PURPOSE
We intended to determine whether dexrazoxane (DZR) is cardioprotective during administration of adjuvant anthracycline-based chemotherapy followed by a 1-year trastuzumab treatment.
METHODS
The medical records of 228 patients who underwent surgical resection and received adjuvant chemotherapy with trastuzumab for human epidermal growth factor receptor type 2 (HER2)-positive breast cancer between January 2010 and December 2014 were reviewed. Approximately 25% of patients received DZR prior to each administration of doxorubicin during doxorubicin with cyclophosphamide (AC) chemotherapy. DZR was not administered during the 1-year trastuzumab maintenance period. Rates of cardiac events (reduction in left ventricular ejection fraction [LVEF] by 10% or more; reduction in absolute LVEF to <45%) and cardiac event-free duration (CFD) were examined. The trastuzumab interruption rate was also assessed.
RESULTS
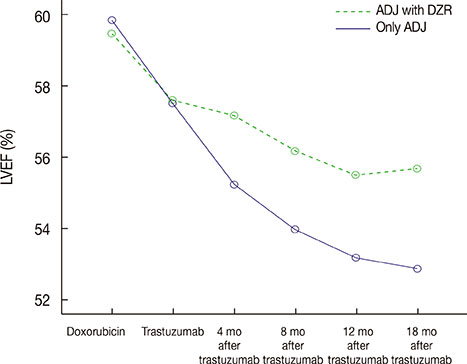
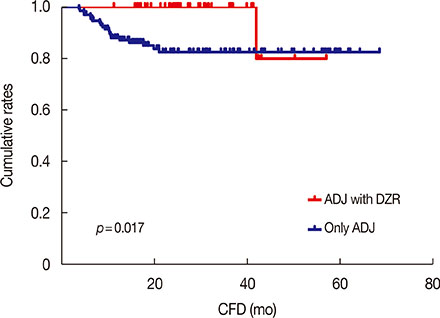
Twelve percent of patients experienced a cardiac event. Repeated-measures analysis of variance for ejection fraction revealed a significant main effect of time, and a significant group (DZR)×time interaction. The group treated with adjuvant chemotherapy and DZR experienced significantly lower frequencies of cardiac events than the adjuvant chemotherapy only group. In multivariate analysis, DZR administration was associated with significantly fewer cardiac events. Moreover, DZR administration was an independent good prognostic factor for CFD. Only one patient (2.3%) experienced early interruption of trastuzumab in the adjuvant chemotherapy with DZR group due to cardiac toxicity, whereas 10 patients (7.6%) experienced a trastuzumab stop event in the adjuvant chemotherapy only group.
CONCLUSION
DZR is cardioprotective in HER2-positive breast cancer patients who received adjuvant chemotherapy with trastuzumab. A large cohort randomized trial is needed to determine if DZR has an effect on trastuzumab interruption and completion of 12-month trastuzumab. Because cardiac toxicity has a significant negative effect on trastuzumab maintenance and quality of life, DZR administration could be considered concomitantly with anthracycline-based adjuvant chemotherapy with trastuzumab.
MeSH Terms
-
Breast Neoplasms*
Breast*
Cardiotoxicity
Chemotherapy, Adjuvant*
Cohort Studies
Cyclophosphamide
Dexrazoxane*
Doxorubicin
Drug Therapy
Humans
Medical Records
Multivariate Analysis
Quality of Life
Receptor, Epidermal Growth Factor
Stroke Volume
Trastuzumab*
Cyclophosphamide
Doxorubicin
Receptor, Epidermal Growth Factor
Trastuzumab
Figure
Reference
-
1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235:177–182.
Article2. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005; 353:1673–1684.
Article3. Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014; 32:3744–3752.
Article4. Ayres LR, de Almeida Campos MS, de Oliveira Gozzo T, Martinez EZ, Ungari AQ, de Andrade JM, et al. Trastuzumab induced cardiotoxicity in HER2 positive breast cancer patients attended in a tertiary hospital. Int J Clin Pharm. 2015; 37:365–372.
Article5. Xue J, Jiang Z, Qi F, Lv S, Zhang S, Wang T, et al. Risk of trastuzumab-related cardiotoxicity in early breast cancer patients: a prospective observational study. J Breast Cancer. 2014; 17:363–369.
Article6. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003; 97:2869–2879.
Article7. Mantarro S, Rossi M, Bonifazi M, D'Amico R, Blandizzi C, La Vecchia C, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med. 2016; 11:123–140.
Article8. Bonifazi M, Franchi M, Rossi M, Moja L, Zambelli A, Zambon A, et al. Trastuzumab-related cardiotoxicity in early breast cancer: a cohort study. Oncologist. 2013; 18:795–801.
Article9. Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004; 351:145–153.
Article10. Speyer J, Wasserheit C. Strategies for reduction of anthracycline cardiac toxicity. Semin Oncol. 1998; 25:525–537.11. Schoenborn CA, Adams PE. Health behaviors of adults: United States, 2005-2007. Vital Health Stat 10. 2010; (245):1–132.12. O'Shaughnessy J, Twelves C, Aapro M. Treatment for anthracycline-pretreated metastatic breast cancer. Oncologist. 2002; 7:Suppl 6. 4–12.13. Lopez M, Vici P, Di Lauro K, Conti F, Paoletti G, Ferraironi A, et al. Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol. 1998; 16:86–92.
Article14. Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997; 15:1318–1332.
Article15. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. NCCN guidelines insights breast cancer, version 1.2016. J Natl Compr Canc Netw. 2015; 13:1475–1485.16. Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 Months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013; 14:741–748.
Article17. Marty M, Espié M, Llombart A, Monnier A, Rapoport BL, Stahalova V, et al. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol. 2006; 17:614–622.
Article18. Zhang S, Meng T, Liu J, Zhang X, Zhang J. Cardiac protective effects of dexrazoxane on animal cardiotoxicity model induced by anthracycline combined with trastuzumab is associated with upregulation of calpain-2. Medicine (Baltimore). 2015; 94:e445.
Article19. Albini A, Cesana E, Donatelli F, Cammarota R, Bucci EO, Baravelli M, et al. Cardio-oncology in targeting the HER receptor family: the puzzle of different cardiotoxicities of HER2 inhibitors. Future Cardiol. 2011; 7:693–704.
Article20. Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005; 23:7811–7819.
Article21. Cha C, Ahn SG, Lee HM, Lee HW, Lee SA, Jeong J. Assessment of adjuvant trastuzumab-associated cardiac toxicity in Korean patients with breast cancer: a single-center analysis. Oncology. 2013; 85:228–234.
Article22. Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008; 26:1231–1238.
Article23. Caussa L, Kirova YM, Gault N, Pierga JY, Savignoni A, Campana F, et al. The acute skin and heart toxicity of a concurrent association of trastuzumab and locoregional breast radiotherapy including internal mammary chain: a single-institution study. Eur J Cancer. 2011; 47:65–73.
Article24. Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015; 372:134–141.
Article25. Leung HW, Chan AL. Trastuzumab-induced cardiotoxicity in elderly women with HER-2-positive breast cancer: a meta-analysis of real-world data. Expert Opin Drug Saf. 2015; 14:1661–1671.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Nine months versus 12 months of adjuvant trastuzumab for patients with HER2-positive breast cancer
- The Predictive Value of Serum HER2/neu for Response to Anthracycline-Based and Trastuzumab-Based Neoadjuvant Chemotherapy
- Impact of Trastuzumab on Ipsilateral Breast Tumor Recurrence for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer after BreastConserving Surgery
- Effective Treatment of Solitary Pituitary Metastasis with Panhypopituitarism in HER2-Positive Breast Cancer by Lapatinib