J Breast Cancer.
2017 Mar;20(1):45-53. 10.4048/jbc.2017.20.1.45.
Clinicopathologic and Prognostic Significance of Transducin-Like Enhancer of Split 1 Protein Expression in Invasive Breast Cancer
- Affiliations
-
- 1Department of Pathology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea. c84103@schmc.ac.kr
- 2Department of Oncology and Hematology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 3Department of Surgery, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 2379398
- DOI: http://doi.org/10.4048/jbc.2017.20.1.45
Abstract
- PURPOSE
Transducin-like enhancer of split 1 (TLE1) is a member of the TLE family of transcriptional co-repressors that control the transcription of a wide range of genes. We investigated the prognostic significance of TLE1 protein expression in breast cancers by using immunohistochemistry and explored the relationship of TLE1 with clinicopathological parameters.
METHODS
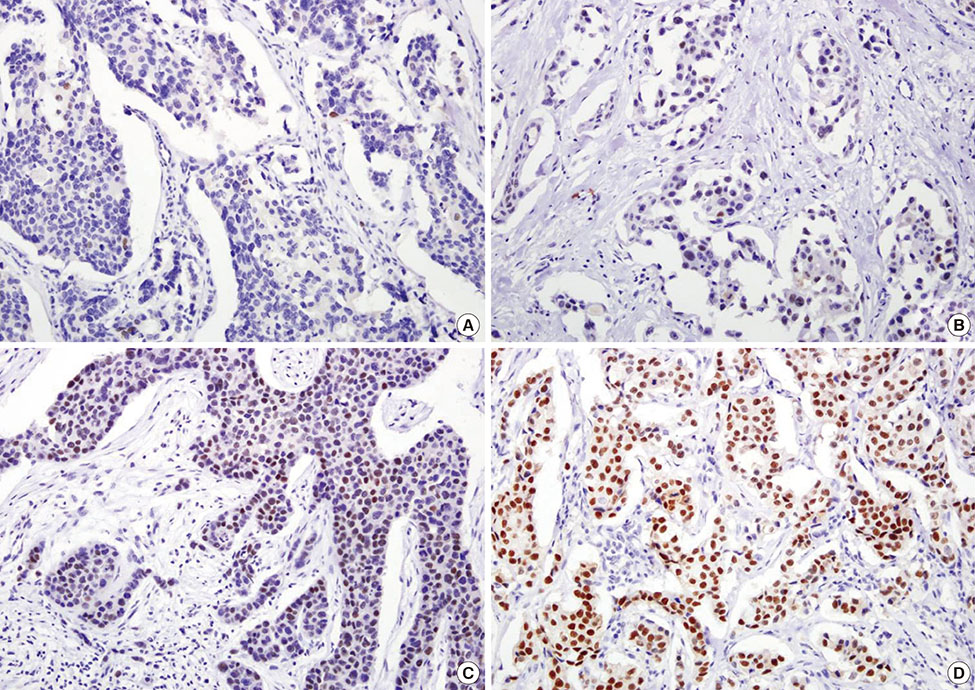
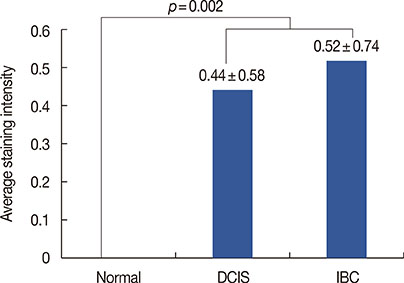
Immunohistochemistry was performed on 456 cases of breast cancer tiled on tissue microarrays. The relationship between TLE1 expression in normal breast specimens and ductal carcinoma in situ (DCIS) was also analyzed.
RESULTS
TLE1 was highly expressed in 57 of 456 (12.5%) carcinoma samples. TLE1 was more frequently expressed in DCIS and invasive breast cancers than in normal breast tissue (p=0.002). High expression of TLE1 significantly correlated with negative lymph node (LN) metastasis (p=0.007), high histologic grade (p<0.001), estrogen receptor negativity (p<0.001), progesterone receptor negativity (p<0.001), human epidermal growth factor receptor 2 (HER2) positivity (p<0.001), and high Ki-67 proliferation index (p<0.001). Based on intrinsic subtypes, high TLE1 expression was strongly associated with HER2+ and triple-negative breast cancers (TNBC) (p<0.001). Survival analysis demonstrated no significant association between TLE1 expression and disease-free survival (DFS) (p=0.167) or overall survival (OS) (p=0.286). In subgroup analyses, no correlation was found between TLE1 expression and DFS or OS according to LN status or intrinsic subtype.
CONCLUSION
High TLE1 expression is significantly associated with the HER2+ and TNBC subtypes. This is the first study documenting immunohistochemical expression of TLE1 in invasive breast cancer and its association with clinicopathological parameters, prognosis, and intrinsic subtype.
Keyword
MeSH Terms
-
Breast Neoplasms*
Breast*
Carcinoma, Intraductal, Noninfiltrating
Co-Repressor Proteins
Disease-Free Survival
Estrogens
Humans
Immunohistochemistry
Lymph Nodes
Neoplasm Metastasis
Prognosis
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Triple Negative Breast Neoplasms
Co-Repressor Proteins
Estrogens
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Figure
Reference
-
1. Min SY, Kim Z, Hur MH, Yoon CS, Park EH, Jung KW, et al. The basic facts of Korean breast cancer in 2013: results of a nationwide survey and breast cancer registry database. J Breast Cancer. 2016; 19:1–7.
Article2. Irshad S, Ellis P, Tutt A. Molecular heterogeneity of triple-negative breast cancer and its clinical implications. Curr Opin Oncol. 2011; 23:566–577.
Article3. Lee HJ, Kim JY, Song IH, Park IA, Yu JH, Ahn JH, et al. High mobility group B1 and N1 (HMGB1 and HMGN1) are associated with tumor-infiltrating lymphocytes in HER2-positive breast cancers. Virchows Arch. 2015; 467:701–709.
Article4. Jang MH, Kim HJ, Kim EJ, Chung YR, Park SY. Expression of epithelial-mesenchymal transition-related markers in triple-negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome. Hum Pathol. 2015; 46:1267–1274.
Article5. Agarwal M, Kumar P, Mathew SJ. The Groucho/transducin-like enhancer of split protein family in animal development. IUBMB Life. 2015; 67:472–481.
Article6. Ciarapica R, Methot L, Tang Y, Lo R, Dali R, Buscarlet M, et al. Prolyl isomerase Pin1 and protein kinase HIPK2 cooperate to promote cortical neurogenesis by suppressing Groucho/TLE: Hes1-mediated inhibition of neuronal differentiation. Cell Death Differ. 2014; 21:321–332.
Article7. Fraga MF, Berdasco M, Ballestar E, Ropero S, Lopez-Nieva P, Lopez-Serra L, et al. Epigenetic inactivation of the Groucho homologue gene TLE1 in hematologic malignancies. Cancer Res. 2008; 68:4116–4122.
Article8. Pretto D, Barco R, Rivera J, Neel N, Gustavson MD, Eid JE. The synovial sarcoma translocation protein SYT-SSX2 recruits beta-catenin to the nucleus and associates with it in an active complex. Oncogene. 2006; 25:3661–3669.
Article9. Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007; 31:240–246.
Article10. Ge C, Wu S, Wang W, Liu Z, Zhang J, Wang Z, et al. miR-942 promotes cancer stem cell-like traits in esophageal squamous cell carcinoma through activation of Wnt/beta-catenin signalling pathway. Oncotarget. 2015; 6:10964–10977.
Article11. Ramasamy S, Saez B, Mukhopadhyay S, Ding D, Ahmed AM, Chen X, et al. Tle1 tumor suppressor negatively regulates inflammation in vivo and modulates NF-kappaB inflammatory pathway. Proc Natl Acad Sci U S A. 2016; 113:1871–1876.
Article12. Valente AL, Tull J, Zhang S. Specificity of TLE1 expression in unclassified high-grade sarcomas for the diagnosis of synovial sarcoma. Appl Immunohistochem Mol Morphol. 2013; 21:408–413.
Article13. Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol. 2009; 22:872–878.
Article14. Seo SW, Lee H, Lee HI, Kim HS. The role of TLE1 in synovial sarcoma. J Orthop Res. 2011; 29:1131–1136.
Article15. Laporte AN, Ji JX, Ma L, Nielsen TO, Brodin BA. Identification of cytotoxic agents disrupting synovial sarcoma oncoprotein interactions by proximity ligation assay. Oncotarget. 2016; 7:34384–34394.
Article16. Lee JH, Son MW, Kim KJ, Oh MH, Cho H, Lee HJ, et al. Prognostic and clinicopathological significance of transducer-like enhancer of split 1 expression in gastric cancer. J Gastric Cancer. 2016; 16:21–27.
Article17. Brunquell C, Biliran H, Jennings S, Ireland SK, Chen R, Ruoslahti E. TLE1 is an anoikis regulator and is downregulated by Bit1 in breast cancer cells. Mol Cancer Res. 2012; 10:1482–1495.
Article18. Allen T, van Tuyl M, Iyengar P, Jothy S, Post M, Tsao MS, et al. Grg1 acts as a lung-specific oncogene in a transgenic mouse model. Cancer Res. 2006; 66:1294–1301.
Article19. Zhang L, Yang L, Liu X, Chen W, Chang L, Chen L, et al. Micro-RNA-657 promotes tumorigenesis in hepatocellular carcinoma by targeting transducin-like enhancer protein 1 through nuclear factor kappa B pathways. Hepatology. 2013; 57:1919–1930.
Article20. Hamidov Z, Altendorf-Hofmann A, Chen Y, Settmacher U, Petersen I, Knösel T. Reduced expression of desmocollin 2 is an independent prognostic biomarker for shorter patients survival in pancreatic ductal adenocarcinoma. J Clin Pathol. 2011; 64:990–994.
Article21. Yao X, Jennings S, Ireland SK, Pham T, Temple B, Davis M, et al. The anoikis effector Bit1 displays tumor suppressive function in lung cancer cells. PLoS One. 2014; 9:e101564.
Article22. Yao X, Ireland SK, Pham T, Temple B, Chen R, Raj MH, et al. TLE1 promotes EMT in A549 lung cancer cells through suppression of E-cadherin. Biochem Biophys Res Commun. 2014; 455:277–284.
Article23. Holmes KA, Hurtado A, Brown GD, Launchbury R, Ross-Innes CS, Hadfield J, et al. Transducin-like enhancer protein 1 mediates estrogen receptor binding and transcriptional activity in breast cancer cells. Proc Natl Acad Sci U S A. 2012; 109:2748–2753.
Article24. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th ed. Lyon: International Agency for Research on Cancer;2012.25. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–168.26. Jang SH, Lee JE, Oh MH, Lee JH, Cho HD, Kim KJ, et al. High EZH2 protein expression is associated with poor overall survival in patients with luminal A breast cancer. J Breast Cancer. 2016; 19:53–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathological Significance of SMAD4 Expression in Breast Cancer
- Clinicopathologic Significance of p53 and c-erbB-2 Protein Expression in Breast Carcinoma
- Clinicopathologic Significance of p53 and c-erbB-2 Protein Expression in Breast Carcinoma
- Downregulation of N-myc and STAT Interactor Protein Predicts Aggressive Tumor Behavior and Poor Prognosis in Invasive Ductal Carcinoma
- Expression of the PTEN Gene Product in the Invasive Cancer of Breast and Its Relationship with Other Prognostic Factors