Healthc Inform Res.
2017 Apr;23(2):94-100. 10.4258/hir.2017.23.2.94.
Effects and Satisfaction of Medical Device Safety Information Reporting System Using Electronic Medical Record
- Affiliations
-
- 1Department of Clinical Trials for Medical Devices, Yonsei University Health System Severance Hospital, Seoul, Korea.
- 2Department of Medical Engineering, Yonsei University College of Medicine, Seoul, Korea. knh@yuhs.ac
- KMID: 2378517
- DOI: http://doi.org/10.4258/hir.2017.23.2.94
Abstract
OBJECTIVES
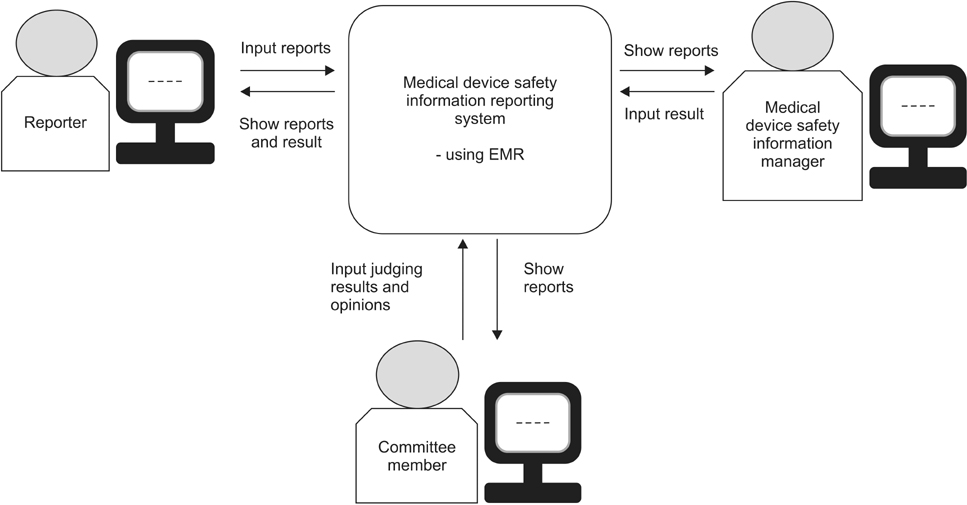
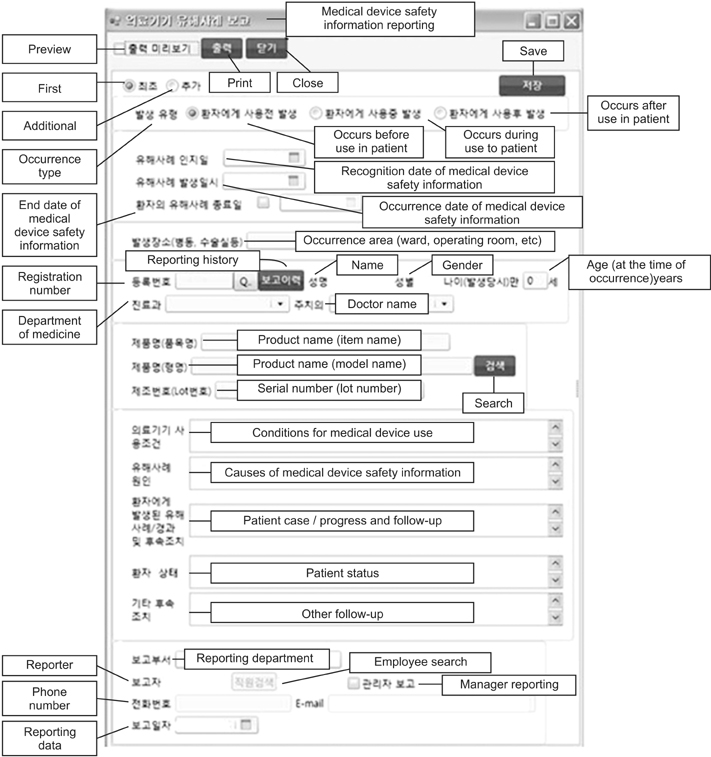
This paper describes an evaluation study on the effectiveness of developing an in-hospital medical device safety information reporting system for managing safety information, including adverse incident data related to medical devices, following the enactment of the Medical Device Act in Korea.
METHODS
Medical device safety information reports were analyzed for 190 cases that took place prior to the application of a medical device safety information reporting system and during a period when the reporting system was used. Also, questionnaires were used to measure the effectiveness of the medical device safety information reporting system. The analysis was based on the questionnaire responses of 15 reporters who submitted reports in both the pre- and post-reporting system periods.
RESULTS
Sixty-two reports were submitted in paper form, but after the system was set up, this number more than doubled to 128 reports in electronic form. In terms of itemized reporting, a total of 45 items were reported. Before the system was used, 23 items had been reported, but this increased to 32 items after the system was put to use. All survey variables of satisfaction received a mean of over 3 points, while positive attitude, potential benefits, and positive benefits all exceeded 4 points, each receiving 4.20, 4.20, and 4.13, respectively. Among the variables, time-consuming and decision-making had the lowest mean values, each receiving 3.53. Satisfaction was found to be high for system quality and user satisfaction, but relatively low for time-consuming and decision-making.
CONCLUSIONS
We were able to verify that effective reporting and monitoring of adverse incidents and the safety of medical devices can be implemented through the establishment of an in-hospital medical device safety information reporting system that can enhance patient safety and medical device risk management.
MeSH Terms
Figure
Cited by 1 articles
-
Feasibility of Computerized Visuomotor Integration System for Visual Field Defects and Spatial Neglect in Poststroke Patients
Hyeon-Taek Hong, Myeong Geun Jeong, Kyoung Tae Kim
Ann Rehabil Med. 2024;48(2):146-154. doi: 10.5535/arm.230028.
Reference
-
1. Korea Health Industry Development Institute. Medical Devices Industry Report. Cheongju: Korea Health Industry Development Institute;2013. p. 41–100.2. Espicom. Worldwide medical market forecasts to 2018. Chichester: Espicom Healthcare Intelligence;2013.3. Beydon L, Ledenmat PY, Soltner C, Lebreton F, Hardin V, Benhamou D, et al. Adverse events with medical devices in anesthesia and intensive care unit patients recorded in the French safety database in 2005-2006. Anesthesiology. 2010; 112(2):364–372.
Article4. Lee KM, Baek NK, Seo JH. A study on the system improvement policy according to the status analysis of medical device control system in Korea. J Korea Saf Manag Sci. 2010; 12(3):37–52.5. Medical Devices Act, Chapter V, Article 31, Law No. 12392. 2014. 07. 29.6. Regulation on the management of safety information as medical device side effect report, MFDS Notification No. 2005-20. 2005. 04. 16.7. Kang CW. There are hospitals that hide Medical side effects, because of prejudice? [Internet]. Seoul: ehealthnews.com;2014. cited at 2014 Dec 21. Available from: http://www.ehealthnews.net.8. Regulation on the management of safety information as medical device side effect report, MFDS Notification No. 2014-91. 2014. 02. 12.9. Delon WH, McLean ER. The DeLone and McLean model of information systems success: a ten-year update. J Manag Inf Syst. 2003; 19(4):9–30.
Article10. Kim MS, Kim JS, Jung IS, Kim YH, Kim HJ. The effectiveness of the error reporting promoting program on the nursing error incidence rate in Korean operating rooms. J Korean Acad Nurs. 2007; 37(2):185–191.
Article11. Weingart SN, Callanan LD, Ship AN, Aronson MD. A physician-based voluntary reporting system for adverse events and medical errors. J Gen Intern Med. 2001; 16(12):809–814.
Article12. Baker KN, McConnell WE. The problems of detecting medication errors in hospitals. Am J Hosp Pharm. 1962; 19:360–369.
Article13. Cohen H. Shrinking medication errors down to size. Nurs Manage. 2001; 32(10):25–30.
Article14. Leape LL. Why should we report adverse incidents? J Eval Clin Pract. 1999; 5(1):1–4.15. Vincent C, Stanhope N, Crowley-Murphy M. Reasons for not reporting adverse incidents: an empirical study. J Eval Clin Pract. 1999; 5(1):13–21.
Article16. Baker HM. Rules outside the rules for administration of medication: a study in New South Wales, Australia. Image J Nurs Sch. 1997; 29(2):155–158.
Article17. Hart GK, Baldwin I, Gutteridge G, Ford J. Adverse incident reporting in intensive care. Anaesth Intensive Care. 1994; 22(5):556–561.
Article18. Jayasuriya JP, Anandaciva S. Compliance with an incident report scheme in anaesthesia. Anaesthesia. 1995; 50(10):846–849.
Article19. Hwang JI, Lee SI, Park HA. Barriers to the operation of patient safety incident reporting systems in Korean general hospitals. Healthc Inform Res. 2012; 18(4):279–286.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Term Effects of Hospital Information System on Nurses' Job Pattern and Satisfaction, and Attitudes Toward HIS
- The Application of Electronic Medical Record and Users' Satisfaction in Ophthalmology
- Medical device adverse effects
- Factors affecting the users'satisfaction on the electronic medical record system
- The Paper-Based Medical Record Compared to the Electronic Medical Record: Documentation and Agreement of Information