J Periodontal Implant Sci.
2017 Apr;47(2):77-85. 10.5051/jpis.2017.47.2.77.
Regenerative capacity of augmented bone in rat calvarial guided bone augmentation model
- Affiliations
-
- 1Division of Applied Oral Sciences, Nihon University Graduate School of Dentistry, Tokyo, Japan.
- 2Department of Periodontology, Nihon University School of Dentistry, Tokyo, Japan. hasuike.akira@nihon-u.ac.jp
- 3Dental Research Center, Nihon University School of Dentistry, Tokyo, Japan.
- 4Private Practice, Kanagawa, Japan.
- KMID: 2377742
- DOI: http://doi.org/10.5051/jpis.2017.47.2.77
Abstract
- PURPOSE
Guided bone regeneration (GBR) is the most widely used technique to regenerate and augment bones. Even though augmented bones (ABs) have been examined histologically in many studies, few studies have been conducted to examine the biological potential of these bones and the healing dynamics following their use. Moreover, whether the bone obtained from the GBR procedure possesses the same functions as the existing autogenous bone is uncertain. In particular, little attention has been paid to the regenerative ability of GBR bone. Therefore, the present study histologically evaluated the regenerative capacity of AB in the occlusive space of a rat guided bone augmentation (GBA) model.
METHODS
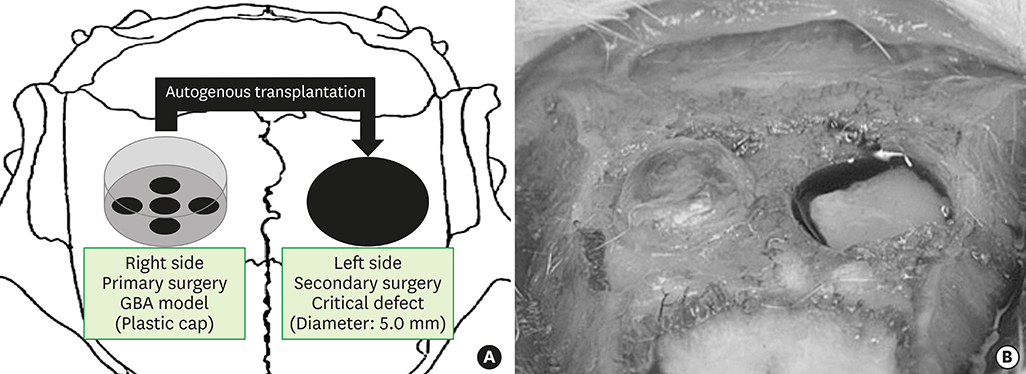
The calvaria of 30 rats were exposed, and plastic caps were placed on the right of the calvaria in 10 of the 30 rats. After a 12-week healing phase, critical-sized calvarial bone defects (diameter: 5.0 mm) were trephined into the dorsal parietal bone on the left of the calvaria. Bone particles were harvested from the AB or the cortical bone (CB) using a bone scraper and transplanted into the critical defects.
RESULTS
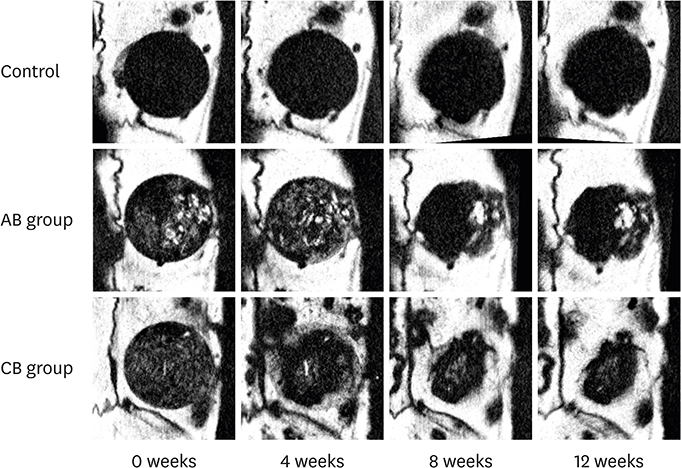
The newly generated bone at the defects' edge was evaluated using micro-computed tomography (micro-CT) and histological sections. In the micro-CT analysis, the radiopacity in both the augmented and the CB groups remained high throughout the observational period. In the histological analysis, the closure rate of the CB was significantly higher than in the AB group. The numbers of cells positive for runt-related transcription factor 2 (Runx2) and tartrate-resistant acid phosphatase (TRAP) in the AB group were larger than in the CB group.
CONCLUSIONS
The regenerative capacity of AB in the occlusive space of the rat GBA model was confirmed. Within the limitations of this study, the regenerative ability of the AB particulate transplant was inferior to that of the CB particulate transplant.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold
Seung-Hwan Kang, Jun-Beom Park, InSoo Kim, Won Lee, Heesung Kim
J Periodontal Implant Sci. 2019;49(4):258-267. doi: 10.5051/jpis.2019.49.4.258.
Reference
-
1. Simion M, Trisi P, Piattelli A. Vertical ridge augmentation using a membrane technique associated with osseointegrated implants. Int J Periodontics Restorative Dent. 1994; 14:496–511.2. Simion M, Jovanovic SA, Trisi P, Scarano A, Piattelli A. Vertical ridge augmentation around dental implants using a membrane technique and autogenous bone or allografts in humans. Int J Periodontics Restorative Dent. 1998; 18:8–23.3. Tinti C, Parma-Benfenati S, Polizzi G. Vertical ridge augmentation: what is the limit? Int J Periodontics Restorative Dent. 1996; 16:220–229.4. Schmid J, Hämmerle CH, Stich H, Lang NP. Supraplant, a novel implant system based on the principle of guided bone generation. A preliminary study in the rabbit. Clin Oral Implants Res. 1991; 2:199–202.
Article5. Linde A, Thorén C, Dahlin C, Sandberg E. Creation of new bone by an osteopromotive membrane technique: an experimental study in rats. J Oral Maxillofac Surg. 1993; 51:892–897.
Article6. Jovanovic SA, Schenk RK, Orsini M, Kenney EB. Supracrestal bone formation around dental implants: an experimental dog study. Int J Oral Maxillofac Implants. 1995; 10:23–31.7. Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982; 9:290–296.
Article8. Nyman S, Gottlow J, Karring T, Lindhe J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol. 1982; 9:257–265.
Article9. Yamada Y, Nanba K, Ito K. Effects of occlusiveness of a titanium cap on bone generation beyond the skeletal envelope in the rabbit calvarium. Clin Oral Implants Res. 2003; 14:455–463.
Article10. Yamada Y, Sato S, Yagi H, Ujiie H, Ezawa S, Ito K. Correlation in the densities of augmented and existing bone in guided bone augmentation. Clin Oral Implants Res. 2012; 23:837–845.
Article11. Schenk RK, Buser D, Hardwick WR, Dahlin C. Healing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandible. Int J Oral Maxillofac Implants. 1994; 9:13–29.12. Kochi G, Sato S, Ebihara H, Hirano J, Arai Y, Ito K. A comparative study of microfocus CT and histomorphometry in the evaluation of bone augmentation in rat calvarium. J Oral Sci. 2010; 52:203–211.
Article13. Oginuma T, Sato S, Udagawa A, Saito Y, Arai Y, Ito K. Autogenous bone with or without hydroxyapatite bone substitute augmentation in rat calvarium within a plastic cap. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114:S107–S113.
Article14. Shino H, Hasuike A, Arai Y, Honda M, Isokawa K, Sato S. Melatonin enhances vertical bone augmentation in rat calvaria secluded spaces. Med Oral Patol Oral Cir Bucal. 2016; 21:e122–e126.
Article15. Wen B, Li Z, Nie R, Liu C, Zhang P, Miron RJ, et al. Influence of biphasic calcium phosphate surfaces coated with Enamel Matrix Derivative on vertical bone growth in an extra-oral rabbit model. Clin Oral Implants Res. 2016; 27:1297–1304.
Article16. Hosoya A, Ninomiya T, Hiraga T, Zhao C, Yoshiba K, Yoshiba N, et al. Alveolar bone regeneration of subcutaneously transplanted rat molar. Bone. 2008; 42:350–357.
Article17. Gruber R, Baron M, Busenlechner D, Kandler B, Fuerst G, Watzek G. Proliferation and osteogenic differentiation of cells from cortical bone cylinders, bone particles from mill, and drilling dust. J Oral Maxillofac Surg. 2005; 63:238–243.
Article18. Urban IA, Lozada JL, Jovanovic SA, Nagursky H, Nagy K. Vertical ridge augmentation with titanium-reinforced, dense-PTFE membranes and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: a prospective case series in 19 patients. Int J Oral Maxillofac Implants. 2014; 29:185–193.
Article19. Urban IA, Nagursky H, Lozada JL. Horizontal ridge augmentation with a resorbable membrane and particulated autogenous bone with or without anorganic bovine bone-derived mineral: a prospective case series in 22 patients. Int J Oral Maxillofac Implants. 2011; 26:404–414.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of biodegradability and tissue regenerative potential of synthetic biodegradable membranes
- The effect of the freeze dried bone allograft and gel/putty type demineralized bone matrix on osseous regeneration in the rat calvarial defects
- Titanium Mesh for Bone Augmentation in Oral Implant Surgery
- Factors Influencing Regeneration of Calvarial Defects in Rats
- The Analysis of Bone regenerative effect with carriers of bone morphogenetic protein in rat calvarial defects