J Korean Med Sci.
2017 Jun;32(6):992-998. 10.3346/jkms.2017.32.6.992.
Effects of Combination Therapy of Alendronate and Hormonal Therapy on Bone Mineral Density in Postmenopausal Korean Women: Multicenter, Randomized Controlled Clinical Trial
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Hallym University College of Medicine, Anyang, Korea.
- 3Department of Obstetrics and Gynecology, Hanyang University School of Medicine, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Chung-Ang University College of Medicine, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. ymchoi@snu.ac.kr
- 6The Institute of Reproductive Medicine and Population, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2377730
- DOI: http://doi.org/10.3346/jkms.2017.32.6.992
Abstract
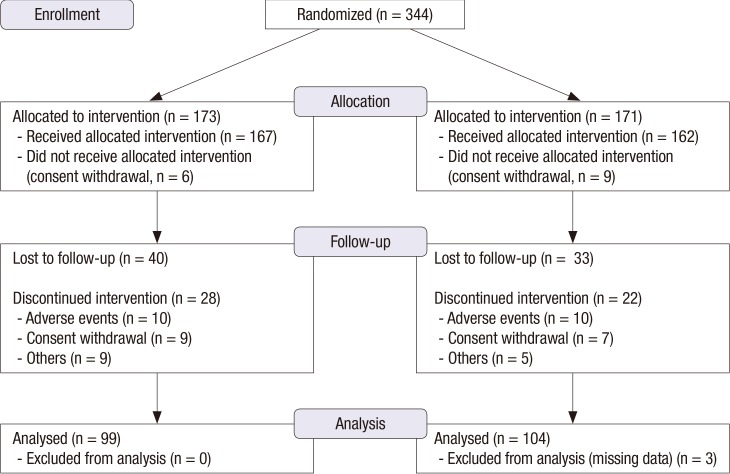
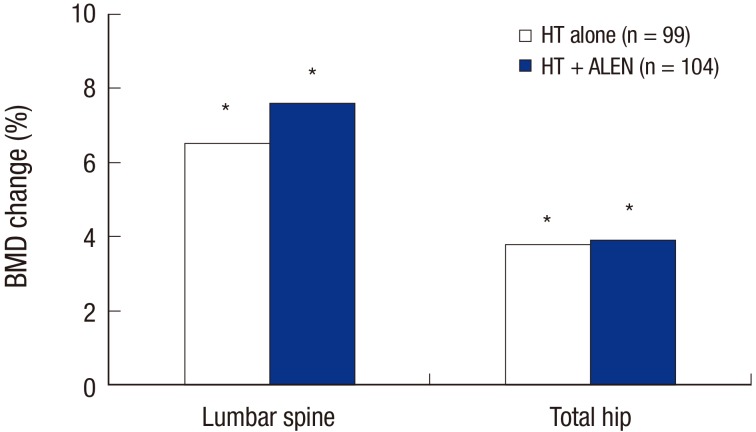
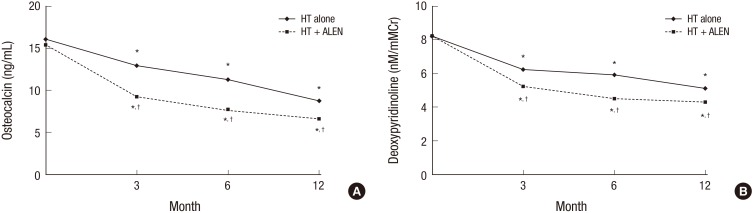
- This study evaluated the effects of combination treatment with alendronate (ALEN) and hormone therapy (HT) on bone mineral density (BMD) in postmenopausal Korean women. This multicenter, randomized, controlled clinical trial enrolled 344 postmenopausal women with low BMD. The women received HT (0.625 mg/day of conjugated equine estrogen and 2.5 mg/day of medroxyprogesterone acetate) alone or in combination with ALEN (10 mg/day) for 1 year. Changes in BMD and biochemical markers of bone turnover were evaluated. Data from 203 women (HT alone, 99; combination treatment, 104) who completed this study were analyzed. BMD at the lumbar spine and total hip increased significantly in both treatment groups after 1 year. There were no significant differences between HT alone vs. the combination of ALEN and HT in mean BMD increase at the lumbar spine (6.9% vs. 7.9%) and total hip (3.7% vs. 3.8%). Combined therapy suppressed serum osteocalcin and urinary deoxypyridinoline to a greater extent than HT alone. In conclusion, compared to HT alone, combination treatment with ALEN and HT for 1 year did not offer a benefit in BMD in postmenopausal Korean women with low BMD.
MeSH Terms
Figure
Reference
-
1. Khosla S, Melton LJ 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res. 2011; 26:441–451. PMID: 20928874.2. Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. The Writing Group for the PEPI. JAMA. 1996; 276:1389–1396. PMID: 8892713.3. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002; 287:2668–2676. PMID: 12020302.4. Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, Mol-Arts M, Kloosterboer L, Mosca L, Christiansen C, et al. The effects of tibolone in older postmenopausal women. N Engl J Med. 2008; 359:697–708. PMID: 18703472.5. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004; 291:1701–1712. PMID: 15082697.6. Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003; 290:1729–1738. PMID: 14519707.7. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996; 348:1535–1541. PMID: 8950879.8. Karpf DB, Shapiro DR, Seeman E, Ensrud KE, Johnston CC Jr, Adami S, Harris ST, Santora AC 2nd, Hirsch LJ, Oppenheimer L, et al. Prevention of nonvertebral fractures by alendronate. A meta-analysis. Alendronate Osteoporosis Treatment Study Groups. JAMA. 1997; 277:1159–1164. PMID: 9087473.9. Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporos Rep. 2003; 1:45–52. PMID: 16036064.10. Khosla S. Update on estrogens and the skeleton. J Clin Endocrinol Metab. 2010; 95:3569–3577. PMID: 20685883.11. Wang Q, Alén M, Nicholson PH, Halleen JM, Alatalo SL, Ohlsson C, Suominen H, Cheng S. Differential effects of sex hormones on peri- and endocortical bone surfaces in pubertal girls. J Clin Endocrinol Metab. 2006; 91:277–282. PMID: 16249282.12. Khastgir G, Studd J, Holland N, Alaghband-Zadeh J, Fox S, Chow J. Anabolic effect of estrogen replacement on bone in postmenopausal women with osteoporosis: histomorphometric evidence in a longitudinal study. J Clin Endocrinol Metab. 2001; 86:289–295. PMID: 11232014.13. Byrjalsen I, Leeming DJ, Qvist P, Christiansen C, Karsdal MA. Bone turnover and bone collagen maturation in osteoporosis: effects of antiresorptive therapies. Osteoporos Int. 2008; 19:339–348. PMID: 17846859.14. Lewiecki EM. Nonresponders to osteoporosis therapy. J Clin Densitom. 2003; 6:307–314. PMID: 14716042.15. Komulainen M, Kröger H, Tuppurainen MT, Heikkinen AM, Honkanen R, Saarikoski S. Identification of early postmenopausal women with no bone response to HRT: results of a five-year clinical trial. Osteoporos Int. 2000; 11:211–218. PMID: 10824236.16. Greenspan SL, Resnick NM, Parker RA. Combination therapy with hormone replacement and alendronate for prevention of bone loss in elderly women: a randomized controlled trial. JAMA. 2003; 289:2525–2533. PMID: 12759324.17. Bone HG, Greenspan SL, McKeever C, Bell N, Davidson M, Downs RW, Emkey R, Meunier PJ, Miller SS, Mulloy AL, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab. 2000; 85:720–726. PMID: 10690882.18. Harris ST, Eriksen EF, Davidson M, Ettinger MP, Moffett Jr AH Jr, Baylink DJ, Crusan CE, Chines AA. Effect of combined risedronate and hormone replacement therapies on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2001; 86:1890–1897. PMID: 11344179.19. Eviö S, Tiitinen A, Laitinen K, Ylikorkala O, Välimäki MJ. Effects of alendronate and hormone replacement therapy, alone and in combination, on bone mass and markers of bone turnover in elderly women with osteoporosis. J Clin Endocrinol Metab. 2004; 89:626–631. PMID: 14764773.20. Wimalawansa SJ. A four-year randomized controlled trial of hormone replacement and bisphosphonate, alone or in combination, in women with postmenopausal osteoporosis. Am J Med. 1998; 104:219–226. PMID: 9552083.21. Lindsay R, Cosman F, Lobo RA, Walsh BW, Harris ST, Reagan JE, Liss CL, Melton ME, Byrnes CA. Addition of alendronate to ongoing hormone replacement therapy in the treatment of osteoporosis: a randomized, controlled clinical trial. J Clin Endocrinol Metab. 1999; 84:3076–3081. PMID: 10487668.22. Tiraş MB, Noyan V, Yildiz A, Yildirim M, Daya S. Effects of alendronate and hormone replacement therapy, alone or in combination, on bone mass in postmenopausal women with osteoporosis: a prospective, randomized study. Hum Reprod. 2000; 15:2087–2092. PMID: 11006178.23. Palomba S, Orio F Jr, Colao A, di Carlo C, Sena T, Lombardi G, Zullo F, Mastrantonio P. Effect of estrogen replacement plus low-dose alendronate treatment on bone density in surgically postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002; 87:1502–1508. PMID: 11932272.24. Tseng LN, Sheu WH, Ho ES, Lan HH, Hu CC, Kao CH. Effects of alendronate combined with hormone replacement therapy on osteoporotic postmenopausal Chinese women. Metabolism. 2006; 55:741–747. PMID: 16713432.25. Min YK, Lee DY, Choi SJ, Kim JH, Choi D, Yoon BK. Effects of adding alendronate to ongoing hormone therapy on bone mineral density in postmenopausal Korean women: a randomized, double-blind, placebo-controlled clinical trial. Menopause. 2013; 20:761–766. PMID: 23403498.26. Kim SW, Park DJ, Park KS, Kim SY, Cho BY, Lee HK, Shin CS. Early changes in biochemical markers of bone turnover predict bone mineral density response to antiresorptive therapy in Korean postmenopausal women with osteoporosis. Endocr J. 2005; 52:667–674. PMID: 16410657.27. Kim JY, Shin KJ, Kim JH, Min YK, Choi D, Lee JH, Yoon BK. The effect of hormone replacement therapy on bone mineral density in Korean postmenopausal women: 2 year prospective cohort study. J Korean Soc Menopause. 1999; 5:150–158.28. Liu JH, Muse KN. The effects of progestins on bone density and bone metabolism in postmenopausal women: a randomized controlled trial. Am J Obstet Gynecol. 2005; 192:1316–1323. PMID: 15846228.29. Seifert-Klauss V, Prior JC. Progesterone and bone: actions promoting bone health in women. J Osteoporos. 2010; 2010:845180. PMID: 21052538.30. Greenspan SL, Emkey RD, Bone HG, Weiss SR, Bell NH, Downs RW, McKeever C, Miller SS, Davidson M, Bolognese MA, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002; 137:875–883. PMID: 12458987.31. Dresner-Pollak R, Parker RA, Poku M, Thompson J, Seibel MJ, Greenspan SL. Biochemical markers of bone turnover reflect femoral bone loss in elderly women. Calcif Tissue Int. 1996; 59:328–333. PMID: 8849397.32. Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD. Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res. 1999; 14:1614–1621. PMID: 10469291.33. Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Bréart G, Meunier PJ, Delmas PD. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–1538. PMID: 8889854.34. Fadanelli ME, Bone HG. Combining bisphosphonates with hormone therapy for postmenopausal osteoporosis. Treat Endocrinol. 2004; 3:361–369. PMID: 15511130.35. Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four-year randomized study. Am J Med. 1995; 99:36–42. PMID: 7598140.36. Greenspan SL, Beck TJ, Resnick NM, Bhattacharya R, Parker RA. Effect of hormone replacement, alendronate, or combination therapy on hip structural geometry: a 3-year, double-blind, placebo-controlled clinical trial. J Bone Miner Res. 2005; 20:1525–1532. PMID: 16059624.37. Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002; 112:281–289. PMID: 11893367.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Bone Mineral Density, Lipid Profiles, and Biochemical Markers after Combination Therapy of Estrogen and Alendronate in Postmenopausal Osteoporosis
- Clinical Usefulness of Alendronate for Osteoporosis in Postmenopausal women
- Combination Therapy of Raloxifene and Alendronate for Treatment of Osteoporosis in Elderly Women
- The short-term effects of combined hormone replacement therapy and alendronate on bone mineral metabolism in postmenopausal women with osteoporosis
- Efficacy and Safety of Monthly 150 mg Oral Ibandronate in Women with Postmenopausal Osteoporosis: A Systematic Review and Meta-analysis of Randomized Controlled Trials