J Dent Rehabil Appl Sci.
2017 Mar;33(1):1-6. 10.14368/jdras.2017.33.1.1.
A proposal of injection points of botulinum toxin into temporal region for chronic migraine
- Affiliations
-
- 1Department of Orofacial Pain and Oral Medicine, Department of Anatomy, College of Dentistry, Yonsei University, Seoul, Republic of Korea. k8756050@yuhs,ac
- KMID: 2377053
- DOI: http://doi.org/10.14368/jdras.2017.33.1.1
Abstract
- Botulinum toxin (BoNT) injections have been used not only in the field of cosmetic surgery such as forehead and eye wrinkle treatment but also in the treatment of chronic migraine, dystonia, spasticity, temporomandibular disorders (TMD). BoNT injections are the only approved therapies to date for prophylactic treatment of chronic migraine patients. Unlike the previously known paralysis of motor neurons, the mechanism of action for migraine is to block the release of non-cholinergic neurotransmitters such as substance P, CGRP, and glutamate, which are associated with peripheral sensitization and neurogenic inflammation in the sensory nerve, it is hypothesized that the signal is blocked. This review focuses on the analgesic effects of BoNT and suggests the direction for the development of injection methods for chronic migraine patients.
Keyword
MeSH Terms
-
Botulinum Toxins*
Dystonia
Forehead
Glutamic Acid
Headache Disorders
Humans
Migraine Disorders*
Motor Neurons
Muscle Spasticity
Neurogenic Inflammation
Neurotransmitter Agents
Paralysis
Substance P
Surgery, Plastic
Temporal Lobe*
Temporomandibular Joint Disorders
Botulinum Toxins
Glutamic Acid
Neurotransmitter Agents
Substance P
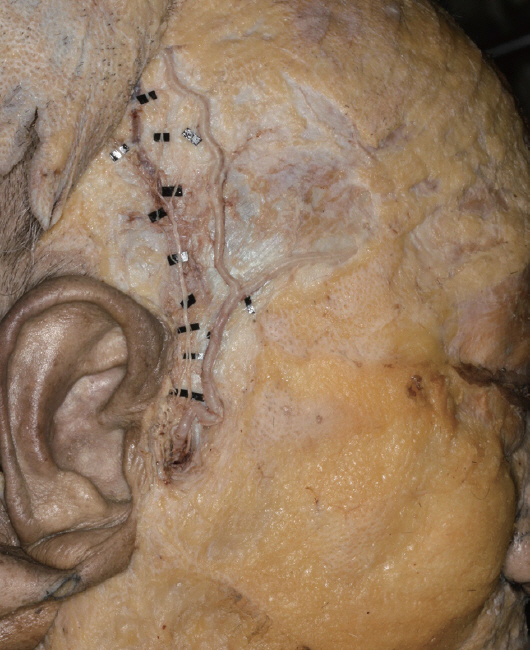
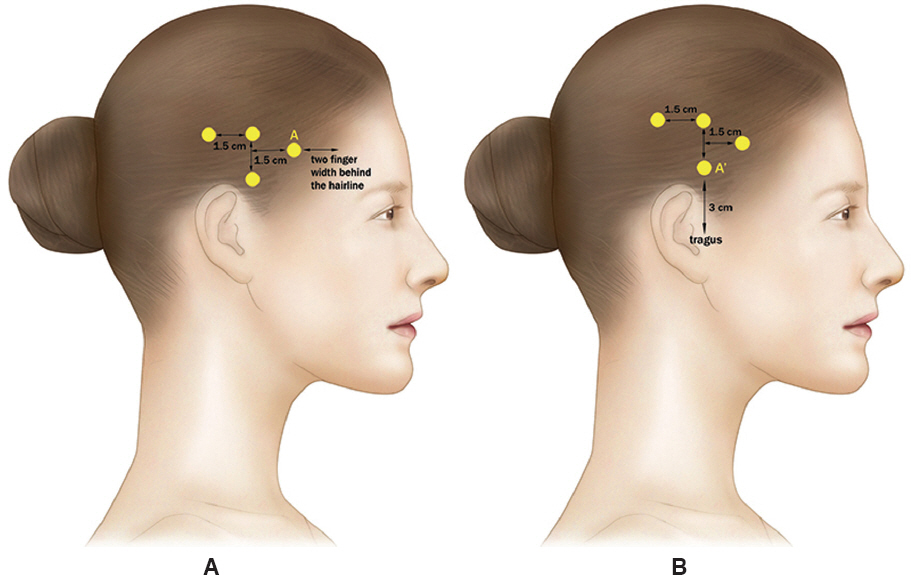
Figure
Reference
-
References
1. Natoli JL, Manack A, Dean B, Butler Q, Turkel CC, Stovner L, Lipton RB. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010; 30:599–609. DOI: 10.1111/j.1468-2982.2009.01941.x. PMID: 19614702.2. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013; 33:629–808.3. U.S. Food and Drug Administration. FDA approves Botox to treat chronic migraine. Available from: https://wayback.archive-it.org/7993/20170112032340/ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm229782.htm. (updated 2017 Feb 1).4. Tsui JK, Eisen A, Mak E, Carruthers J, Scott A, Calne DB. A pilot study on the use of botulinum toxin in spasmodic torticollis. Can J Neurol Sci. 1985; 12:314–6. DOI: 10.1017/S031716710003540X. PMID: 4084867.5. Wheeler A, Smith HS. Botulinum toxins: mechanisms of action, antinociception and clinical applications. Toxicology. 2013; 306:124–46. DOI: 10.1016/j.tox.2013.02.006. PMID: 23435179.6. Durham PL, Cady R. Insights into the mechanism of onabotulinumtoxinA in chronic migraine. Headache. 2011; 51:1573–7. DOI: 10.1111/j.1526-4610.2011.02022.x. PMID: 22082429. PMCID: PMC3306767.7. Filippi GM, Errico P, Santarelli R, Bagolini B, Manni E. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol. 1993; 113:400–4. DOI: 10.3109/00016489309135834. PMID: 8390772.8. Ishikawa H, Mitsui Y, Yoshitomi T, Mashimo K, Aoki S, Mukuno K, Shimizu K. Presynaptic effects of botulinum toxin type A on the neuronally evoked response of albino and pigmented rabbit iris sphincter and dilator muscles. Nippon Ganka Gakkai Zasshi. 2001; 105:218–22. DOI: 10.1016/s0021-5155(01)00384-7.9. Rosales RL, Arimura K, Takenaga S, Osame M. Extrafusal and intrafusal muscle effects in experimental botulinum toxin-A injection. Muscle Nerve. 1996; 19:488–96. DOI: 10.1002/(SICI)1097-4598(199604)19:4<488::AID-MUS9>3.0.CO;2-8.10. Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005; 26:785–93. DOI: 10.1016/j.neuro.2005.01.017. PMID: 16002144.11. Filipović B, Matak I, Bach-Rojecky L, Lackovi Z. Central action of peripherally applied botulinum toxin type A on pain and dural protein extravasation in rat model of trigeminal neuropathy. PloS One. 2012; 7:e29803. DOI: 10.1371/journal.pone.0029803. PMID: 22238656. PMCID: PMC3251614.12. Matak I, Bach-Rojecky L, Filipović B, Lacković Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011; 186:201–7. DOI: 10.1016/j.neuroscience.2011.04.026. PMID: 21539899.13. Baumel JJ, Vanderheiden JP, McElenney JE. The auriculotemporal nerve of man. Am J Anat. 1971; 130:431–40. DOI: 10.1002/aja.1001300405. PMID: 5581228.14. Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010; 50:1406–18. DOI: 10.1111/j.1526-4610.2010.01766.x. PMID: 20958294.15. Ashkenazi A, Blumenfeld A. OnabotulinumtoxinA for the treatment of headache. Headache. 2013; 53(Suppl2):54–61. DOI: 10.1111/head.12185. PMID: 24024603.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Proposal to Prevent the "Mephisto Sign" Side Effect of Botulinum Toxin Type A Injection in Chronic Migraine
- Botulinum Toxin Type A Therapy in Chronic Headache Patients
- Botulinum toxin type A therapy in cluster headache: A case report
- The Complications Developed after Repeated Botulinum Toxin Injection
- The Effect of Botulinum Toxin Chemodenervation in Chronic Paralytic Strabismus