J Korean Soc Radiol.
2017 May;76(5):326-332. 10.3348/jksr.2017.76.5.326.
Morphologic Features of Puncture Sites after ExoSeal Vascular Closure Device Implantation: Changes on Follow-Up Computed Tomography
- Affiliations
-
- 1Department of Radiology, Pusan National University Yangsan Hospital, Yangsan, Korea. junwb73@pnuyh.co.kr
- 2Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea.
- KMID: 2377035
- DOI: http://doi.org/10.3348/jksr.2017.76.5.326
Abstract
- PURPOSE
The study aimed to evaluate the morphologic changes in transarterial chemoembolization (TACE) puncture sites implanted with an ExoSeal vascular closure device (VCD) using follow-up computed tomography (CT).
MATERIALS AND METHODS
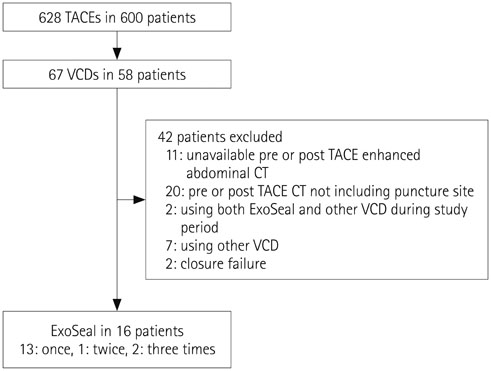
16 patients who used ExoSeal VCD after TACE were enrolled. Using CT images, the diameters and anterior wall thicknesses of the puncture sites in the common femoral artery (CFA) were compared with those of the contralateral CFA before TACE, at 1 month after every TACE session, and at the final follow-up period. The rates of complications were also evaluated.
RESULTS
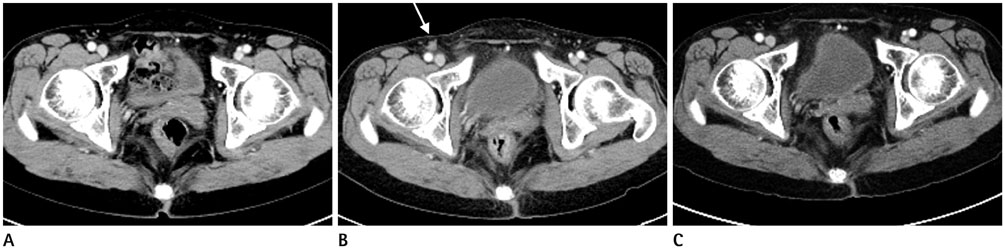
There were no puncture- or VCD-related complications. Follow-up CT images of the CFA's of patients who used ExoSeal VCDs showed eccentric vascular wall thickening with soft-tissue densities considered to be hemostatic plugs. Final follow-up CT images (mean, 616 days; range, 95-1106 days) revealed partial or complete resorption of the hemostatic plugs. The CFA puncture site diameters did not differ statistically from those of the contralateral CFA on the final follow-up CT (p > 0.05), regardless of the number of VCDs used.
CONCLUSION
Follow-up CT images of patients who used ExoSeal VCDs showed no significant vascular stenosis or significant vessel wall thickening.
MeSH Terms
Figure
Reference
-
1. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002; 35:1164–1171.2. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003; 37:429–442.3. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002; 359:1734–1739.4. Marelli L, Shusang V, Senzolo M, Cholongitas E, Goode A, Yu D, et al. Repeated courses of transarterial embolization with polyvinyl alcohol particles: ‘long life elixir’ in a cirrhotic patient with unresectable hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2007; 19:329–332.5. Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002; 13(9 Pt 2):S211–S221.6. Tavris DR, Gallauresi BA, Lin B, Rich SE, Shaw RE, Weintraub WS, et al. Risk of local adverse events following cardiac catheterization by hemostasis device use and gender. J Invasive Cardiol. 2004; 16:459–464.7. Schwartz BG, Burstein S, Economides C, Kloner RA, Shavelle DM, Mayeda GS. Review of vascular closure devices. J Invasive Cardiol. 2010; 22:599–607.8. Lasic Z, Nikolsky E, Kesanakurthy S, Dangas G. Vascular closure devices: a review of their use after invasive procedures. Am J Cardiovasc Drugs. 2005; 5:185–200.9. Wong SC, Bachinsky W, Cambier P, Stoler R, Aji J, Rogers JH, et al. A randomized comparison of a novel bioabsorbable vascular closure device versus manual compression in the achievement of hemostasis after percutaneous femoral procedures: the ECLIPSE (Ensure's Vascular Closure Device Speeds Hemostasis Trial). JACC Cardiovasc Interv. 2009; 2:785–793.10. Chung J, Lee DW, Kwon OS, Kim BS, Shin YS. Angio-Seal™ Evolution™ versus manual compression for common femoral artery puncture in neurovascular diagnostic angiography: a prospective, non-randomized study. J Korean Neurosurg Soc. 2011; 49:153–156.11. Upponi SS, Ganeshan AG, Warakaulle DR, Phillips-Hughes J, Boardman P, Uberoi R. Angioseal versus manual compression for haemostasis following peripheral vascular diagnostic and interventional procedures--a randomized controlled trial. Eur J Radiol. 2007; 61:332–334.12. Hieb RA, Neisen MJ, Hohenwalter EJ, Molnar JA, Rilling WS. Safety and effectiveness of repeat arterial closure using the AngioSeal device in patients with hepatic malignancy. J Vasc Interv Radiol. 2008; 19:1704–1708.13. McEvoy SH, McCarthy CJ, Lavelle LP, Moran DE, Cantwell CP, Skehan SJ, et al. Hepatocellular carcinoma: illustrated guide to systematic radiologic diagnosis and staging according to guidelines of the American Association for the Study of Liver Diseases. Radiographics. 2013; 33:1653–1668.14. Boschewitz JM, Andersson M, Naehle CP, Schild HH, Wilhelm K, Meyer C. Retrospective evaluation of safety and effectiveness of the EXOSEAL vascular closure device for single vascular closure and closure after repeat puncture in diagnostic and interventional radiology: single-center experience. J Vasc Interv Radiol. 2013; 24:698–702.15. Choo HJ, Jeong HW, Park JY, Jin SC, Kim ST, Seo JH, et al. Ultrasonographic features of vascular closure devices: initial and 6-month follow-up results. Ultrasonography. 2014; 33:283–290.16. Biancari F, D'Andrea V, Di Marco C, Savino G, Tiozzo V, Catania A. Meta-analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. Am Heart J. 2010; 159:518–531.17. Eggebrecht H, Haude M, Woertgen U, Schmermund A, von Birgelen C, Naber C, et al. Systematic use of a collagen-based vascular closure device immediately after cardiac catheterization procedures in 1,317 consecutive patients. Catheter Cardiovasc Interv. 2002; 57:486–495.18. Krishnasamy VP, Hagar MJ, Scher DJ, Sanogo ML, Gabriel GE, Sarin SN. Vascular closure devices: technical tips, complications, and management. Tech Vasc Interv Radiol. 2015; 18:100–112.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Could real-time ultrasonography guidance be useful for the effective deployment of FemoSeal in common femoral arteriotomy?
- Safety and Effectiveness of a Circumferential Clip-Based Vascular Closure Device for Hemostasis in Off-Label Applications: Comparison with Standard Applications
- Prospective Comparison of Collagen Plug (Angio-SealTM) and Suture-Mediated (the Closer STM) Closure Devices at Femoral Access Sites
- Comprehensive understanding of atrial septal defects by imaging studies for successful transcatheter closure
- Echocardiographic Evaluation of Atrial Septal Defect Device Closure