J Korean Soc Radiol.
2016 Sep;75(3):171-176. 10.3348/jksr.2016.75.3.171.
Safety and Effectiveness of a Circumferential Clip-Based Vascular Closure Device for Hemostasis in Off-Label Applications: Comparison with Standard Applications
- Affiliations
-
- 1Department of Radiology, Pusan National University School of Medicine, Pusan National University Hospital, Busan, Korea. radkim@nate.com
- 2Department of Radiology, Pusan National University School of Medicine, Yangsan Pusan National University Hospital, Yangsan, Korea.
- KMID: 2349321
- DOI: http://doi.org/10.3348/jksr.2016.75.3.171
Abstract
- PURPOSE
We investigated the efficacy and safety of a circumferential nitinol clip based arterial closure device following arteriotomy, especially in off-label applications.
MATERIALS AND METHODS
Consecutive patients who underwent the procedure with arteriotomy from January 2011 to February 2014 were included in this study. We defined standard use as the use of StarClose for retrograde puncture of the common femoral artery (CFA) and off-label use as the use of StarClose for retrograde puncture of the superficial femoral artery (SFA), antegrade puncture of the CFA or SFA, puncture of the brachial artery or puncture of the vascular graft. The procedures performed included percutaneous transluminal angioplasty and thrombolysis. Technical success was defined as complete hemostasis achieved within 3 minute after the closure. Complications, and laboratory findings associated with coagulation function, were also investigated.
RESULTS
There were 146 cases of standard applications and 111 cases of off-label applications. Technical success was achieved in all cases. The off-label group comprised the use of StarClose for retrograde puncture of the SFA (n = 19), antegrade puncture of the CFA or SFA (n = 74), brachial artery puncture (n = 5), larger sheath than 6 Fr (n = 7) and vascular graft puncture (n = 6). Minor complications were noted in both groups (standard group: 7.5%, off-label group: 2.7%).
CONCLUSION
Off-label use of StarClose is safe and feasible.
MeSH Terms
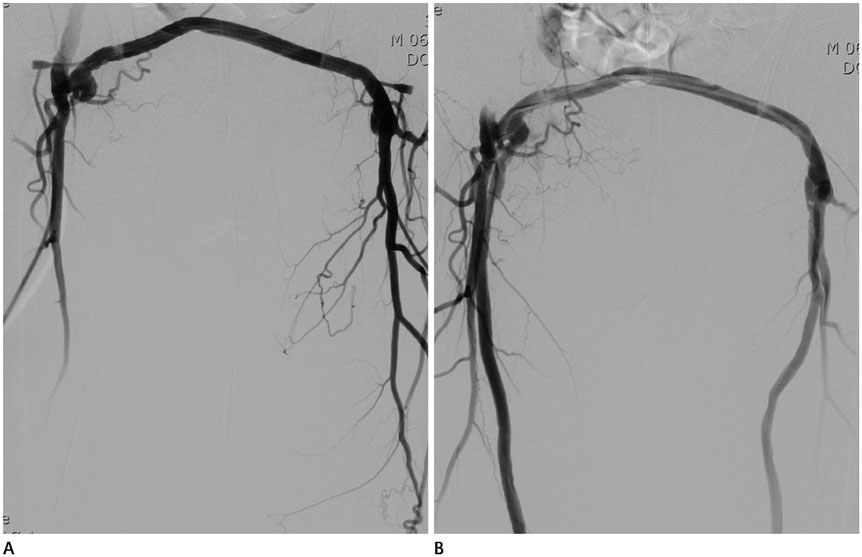
Figure
Reference
-
1. Williams RE, Angel CY, Bourkaib R, Brenot P, Commeau P, Fisher RK, et al. Multicenter safety and efficacy analysis of assisted closure after antegrade arterial punctures using the StarClose device. J Endovasc Ther. 2007; 14:498–505.2. Spiliopoulos S, Katsanos K, Karnabatidis D, Diamantopoulos A, Nikolaos C, Siablis D. Safety and efficacy of the StarClose vascular closure device in more than 1000 consecutive peripheral angioplasty procedures. J Endovasc Ther. 2011; 18:435–443.3. Puggioni A, Boesmans E, Deloose K, Peeters P, Bosiers M. Use of StarClose for brachial artery closure after percutaneous endovascular interventions. Vascular. 2008; 16:85–90.4. Hoffer EK, Bloch RD. Percutaneous arterial closure devices. J Vasc Interv Radiol. 2003; 14:865–885.5. Hwangbo L, Kwak M, Lee S, Kim CW. Hemostatic efficacy and safety of a novel mechanical compression device for femoral arteriotomy. J Korean Soc Radiol. 2015; 72:329–334.6. Prajapati HJ, Rafi S, Edalat F, Kooby DA, Kim HS. Safety and efficacy of a circumferential clip-based vascular closure device in cirrhotic and coagulopathic patients with hepatocellular carcinoma after doxorubicin drug-eluting beads transarterial chemoembolization. Cardiovasc Intervent Radiol. 2014; 37:664–670.7. Imam A, Carter RM, Phillips-Hughes J, Boardman P, Uberoi R. StarClose vascular closure device: prospective study on 222 deployments in an interventional radiology practice. Cardiovasc Intervent Radiol. 2007; 30:738–742.8. Hermiller JB, Simonton C, Hinohara T, Lee D, Cannon L, Mooney M, et al. The StarClose Vascular Closure System: interventional results from the CLIP study. Catheter Cardiovasc Interv. 2006; 68:677–683.9. Maxien D, Behrends B, Eberhardt KM, Saam T, Thieme SF, Reiser MF, et al. Evaluation of the 6-F ExoSeal vascular closure device in antegrade femoral artery punctures. J Endovasc Ther. 2012; 19:836–843.10. Looby S, Keeling AN, McErlean A, Given MF, Geoghegan T, Lee MJ. Efficacy and safety of the angioseal vascular closure device post antegrade puncture. Cardiovasc Intervent Radiol. 2008; 31:558–562.11. Eisenberg RL, Mani RL, McDonald EJ. The complication rate of catheter angiography by direct puncture through aorto-femoral bypass grafts. AJR Am J Roentgenol. 1976; 126:814–816.12. AbuRahma AF, Robinson PA, Boland JP. Safety of arteriography by direct puncture of a vascular prosthesis. Am J Surg. 1992; 164:233–236.13. Katoh H, Nozue T, Michishita I. Direct puncture of the prosthetic bypass graft in the treatment of critical limb ischemia patient undergoing prior axillo-femoral bypass. Cardiovasc Interv Ther. 2013; 28:123–127.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Could real-time ultrasonography guidance be useful for the effective deployment of FemoSeal in common femoral arteriotomy?
- Endoscopic Management of Gastrointestinal Leaks and Bleeding with the Over-the-Scope Clip: A Prospective Study
- Surgical Treatment of Acute Arterial Occlusion after Applying the Vascular Closure Device for Hemostasis of Femoral Arteriotomy in Vascular Intervention
- Endoscopic hemostasis using an over-the-scope clip for massive bleeding after percutaneous endoscopic gastrostomy removal: a case report
- Delayed Vascular Claudication Following Diagnostic Cerebral Angiography: A Rare Complication of the AngioSeal Arteriotomy Closure Device