Nat Prod Sci.
2017 Mar;23(1):9-15. 10.20307/nps.2017.23.1.9.
Optimization of Extraction Conditions for Active Compounds of Herbal Medicinal Formula, DF, by Response Surface Methodology
- Affiliations
-
- 1College of Pharmacy, Kangwon National University, Chuncheon 24341, Korea. heejyang@kangwon.ac.kr
- 2College of Pharmacy, Yonsei Institute of Pharmaceutical Science, Yonsei University, Incheon 21983, Korea.
- 3Department of Pharmacology, School of Medicine, Institute of Health Sciences, Gyeongsang National University, Jinju 52727, Korea.
- 4Department of Formula Sciences, College of Korean Medicine, Dong-Eui University, Busan 47227, Korea.
- 5Department of Microbiology, College of Medicine, Chung-Ang University, Seoul 06974, Korea.
- KMID: 2376493
- DOI: http://doi.org/10.20307/nps.2017.23.1.9
Abstract
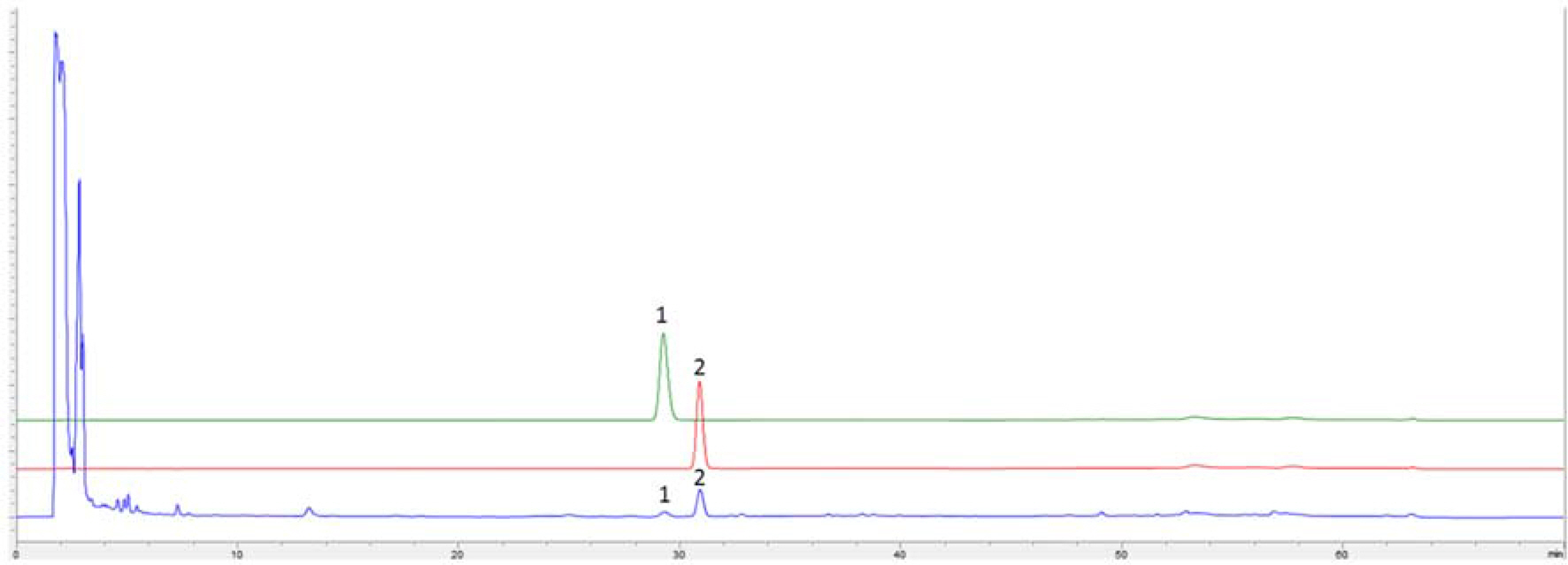
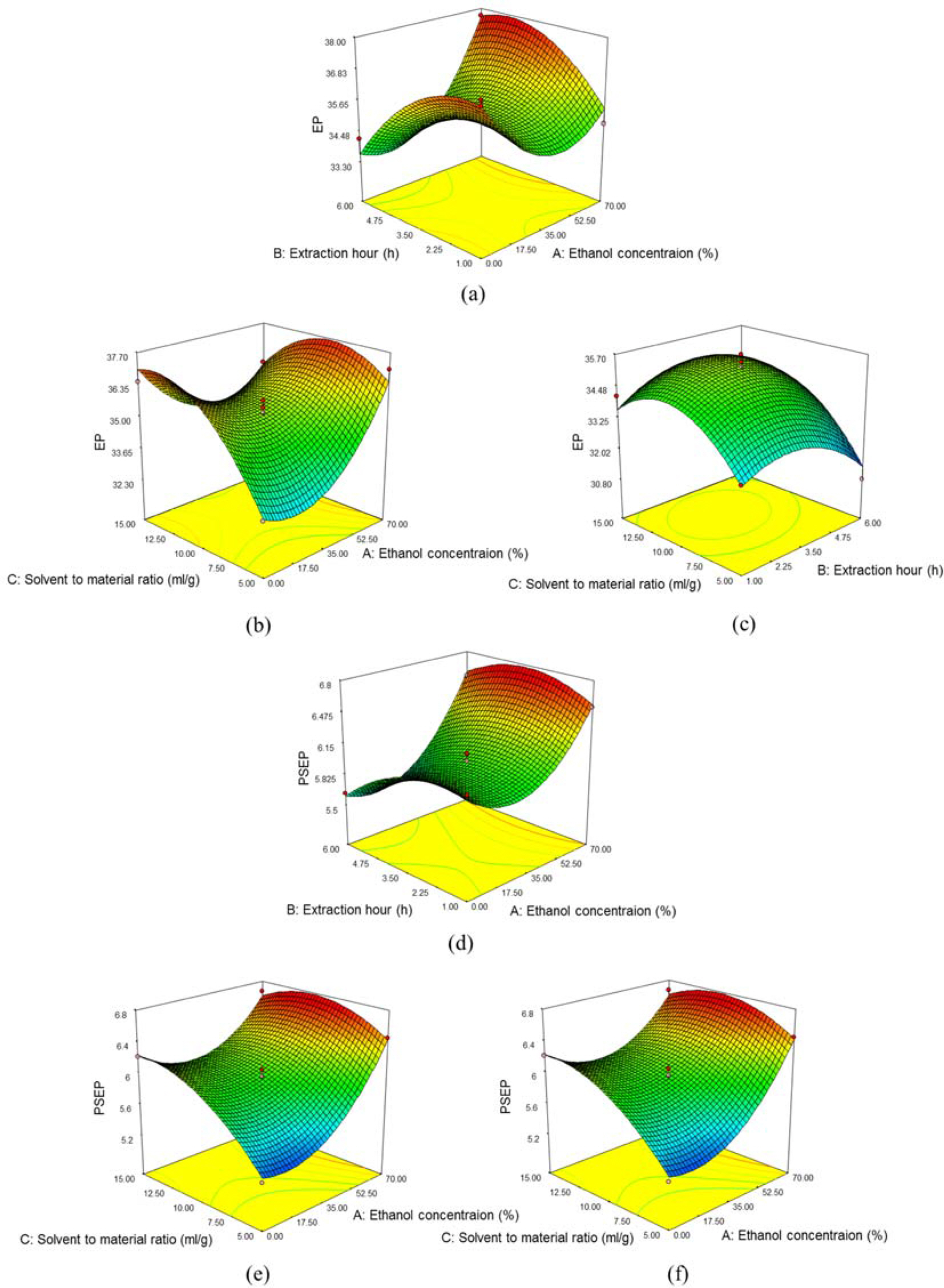
- DF formula is comprised of three traditional herbs, Ephedra intermedia, Rheum palmatum and Lithospermum erythrorhizon, and locally used for treating of the metabolic diseases, such as obesity and diabetes in Korea. We tried to optimize the extraction conditions of two major components, (−)-ephedrine and (+)-pseudoephedrine, in DF formula using response surface methodology with Box-Behnken design (BBD). The experimental conditions with 70% for EtOH concentrations, 4.8 hour for extraction hours and 8.7 times for the solvent to material ratio were suggested for the optimized extraction of DF formula with the highest amounts of (−)-ephedrine and (+)-pseudoephedrine in the designed model.
Keyword
MeSH Terms
Figure
Reference
-
References
(1). Uzuner H.., Bauer R.., Fan T. P.., Guo D. A.., Dias A.., El-Nezami H.., Efferth T.., Williamson E. M.., Heinrich M.., Robinson N.., Hylands P. J.., Hendry B. M.., Cheng Y. C.., Xu Q. J.Ethnopharmacol. 2012. 140:458–468.(2). Ferreira S. L.., Bruns R. E.., da Silva E. G.., Dos Santos W. N.., Quintella C. M.., David J. M.., de Andrade J. B.., Breitkreitz M. C.., Jardim I. C.., Neto B. B. J.Chromatogr. A. 2007. 1158:2–14.(3). Roman M. C. J.AOAC Int. 2004. 87:1–14.(4). Ma G.., Bavadekar S. A.., Davis Y. M.., Lalchandani S. G.., Nagmani R.., Schaneberg B. T.., Khan I. A.., Feller D. R. J.Pharmacol. Exp. Ther. 2007. 322:214–221.(5). Fleming R. M.Expert Opin. Drug Saf. 2008. 7:749–759.
Article(6). Stohs S. J.., Badmaev V.Phytother. Res. 2016. 30:732–740.(7). Pittler M. H.., Schmidt K.., Ernst E.Obes. Rev. 2005. 6:93–111.(8). Stohs S. J.Plast. Reconstr. Surg. 2013. 132:876e–877e.(9). Aichner D.., Ganzera M.Talanta. 2015. 144:1239–1244.(10). Wang H.., Song H.., Yue J.., Li J.., Hou Y. B.., Deng J. L.Cochrane Database Syst. Rev. 2012. CD008000.(11). Papageorgiou V. P.., Assimopoulou A. N.., Ballis A. C.Curr. Med. Chem. 2008. 15:3248–3267.(12). Hu Y.., Jiang Z.., Leung K. S.., Zhao Z.Anal. Chim. Acta. 2006. 577:26–31.(13). Aslan, N; Cebeci Y.Fuel. 2007. 86:90–97.(14). Schaneberg B. T.., Crockett S.., Bedir E.., Khan I. A.Phytochemistry. 2003. 62:911–918.(15). Ichikawa M.., Udayama M.., Imamura K.., Shiraishi S.., Matsuura H.Chem. Pharm. Bull. 2003. 51:635–639.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimization of Extraction Condition of Hesperidin in Citrus unshiu Peels using Response Surface Methodology
- Optimization of the Extraction of Polyphenols and Flavonoids from Argania spinosa Leaves using Response Surface Methodology
- Antioxidant Activity and Phenolic Content of Different Parts of Lotus and Optimization of Extraction Condition using Response Surface Methodology
- Effect of Extraction Conditions of Green Tea on Antioxidant Activity and EGCG Content: Optimization using Response Surface Methodology
- Optimization of Cultivation and Extraction Conditions of Pupae-Cordyceps for Cordycepin Production