Nat Prod Sci.
2016 Dec;22(4):270-274. 10.20307/nps.2016.22.4.270.
Effect of Extraction Conditions of Green Tea on Antioxidant Activity and EGCG Content: Optimization using Response Surface Methodology
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 28644, Korea. mklee@chungbuk.ac.kr
- KMID: 2366695
- DOI: http://doi.org/10.20307/nps.2016.22.4.270
Abstract
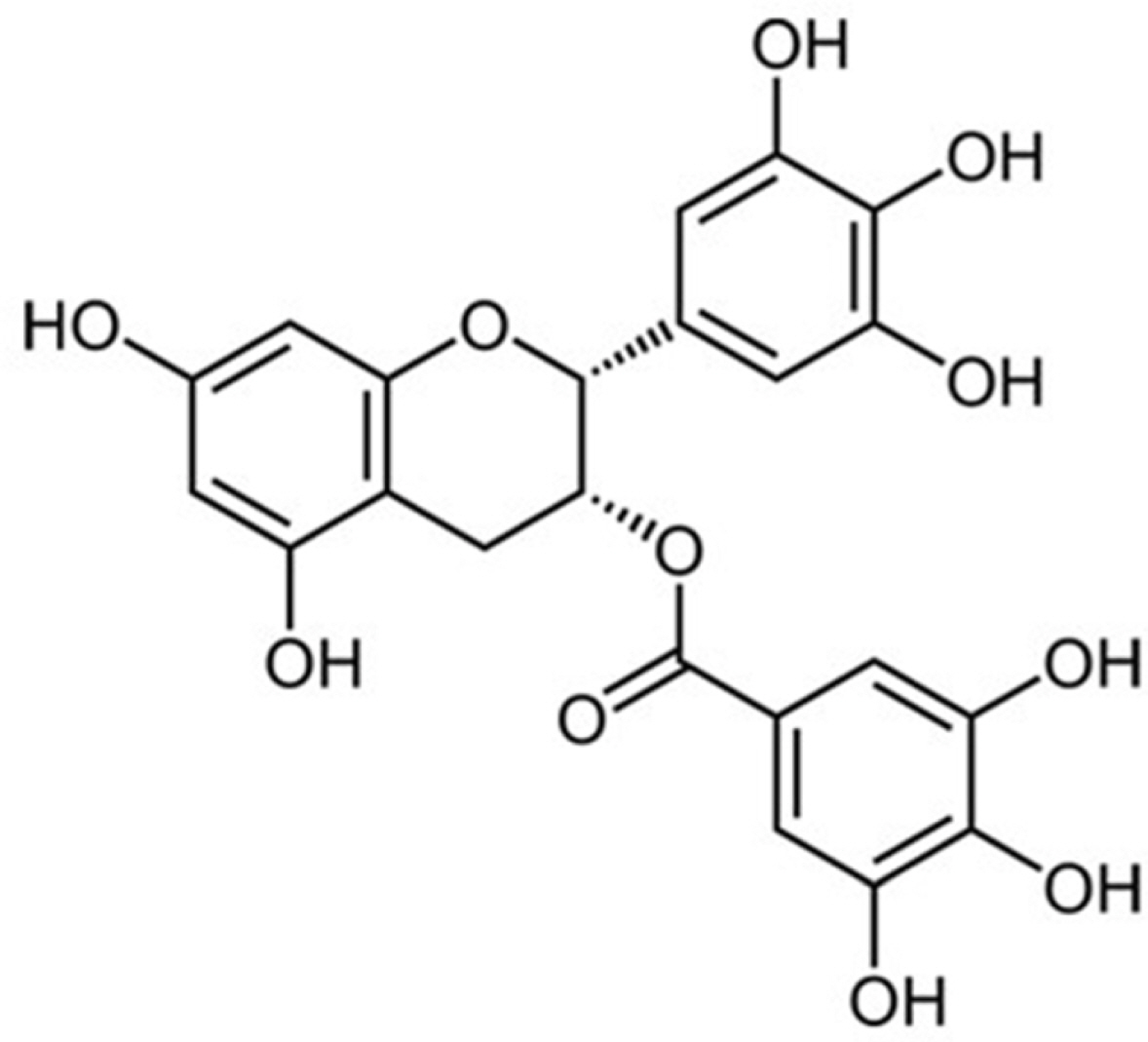
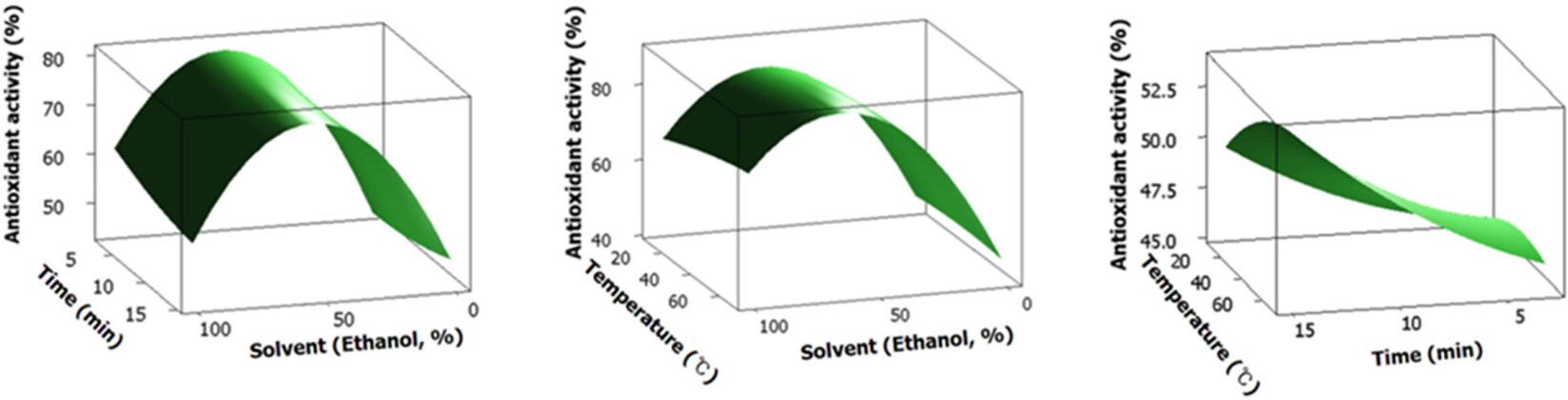
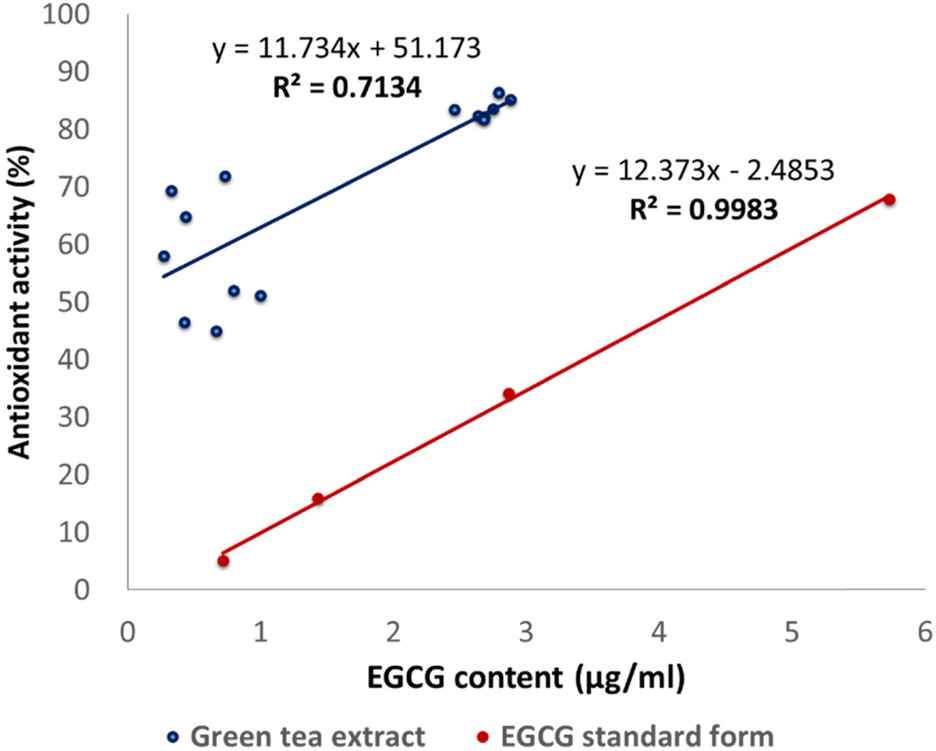
- Green tea, the leaves of Camellia sinsneis (Theaceae), is generally acknowledged as the most consumed beverage with multiple pharmacological functions including antioxidant activity. This study was performed to analyze the effect of extraction conditions of green tea on its antioxidant effects using DPPH assay. Three extraction factors such as extraction solvent (EtOH, 0 - 100%), extraction time (3 - 15 min) and extraction temperature (10 - 70℃) were analyzed and optimized extraction condition for antioxidant activity of green tea extract (GTE) was determined using response surface methodology with three-level-three-factor Box-Behnken design (BBD). Regression analysis showed a good fit of data and the optimal conditions of extraction were found to be 57.7% EtOH, 15 min and 70℃. Under this condition, antioxidant activity of experimental data was 88.4% which was almost fit to the ideal value of 88.6%. As epigallocatechin gallate (EGCG) is known for the major ingredient for antioxidant activity of green tea, we investigated the effect of EGCG on antioxidant activity of GTE. EGCG showed antioxidant activity with the IC50 value of 4.2 µg/ml and a positive correlation was observed between EGCG content and the antioxidant activity of GTE with R2 = 0.7134. Interestingly, however, GTE with 50 - 70% antioxidant activity contain less than 1.0 µg/ml of EGCG, which is much lower than IC50 value of EGCG. Therefore, we suppose that EGCG together with other constituents contribute to antioxidant activity of GTE. Taken together, these results suggest that green tea is more beneficial than EGCG alone for antioxidant ability and optimal extraction condition of green tea will be useful for the development of food and pharmaceutical applications.
Keyword
Figure
Reference
-
References
(1). Zhang L.., Pang E.., Loo R. R.., Rao J.., Go V. L.., Loo J. A.., Lu Q. Y.Proteomics. 2011. 11:4638–4647.(2). Bornhoeft J.., Castaneda D.., Nemoseck T.., Wang P.., Henning S. M.., Hong M. Y. J.Med. Food. 2012. 15:726–732.(3). Rickman C.., Lyer A.., Chan V.., Brown L.Cur. Pharm. Biotechnol. 2010. 11:881–886.(4). Ramadan G.., El-Beih N. M.., Abd El-Ghffar E. A.Br. J. Nutr. 2009. 102:1611–1619.(5). Du G. J.., Zhang Z.., Wen X.D.., Yu C.., Calway T.., Yuan C. S.., Wang C. Z.Nutrients. 2012. 4:1679–1691.(6). Zhong Y.., Chiou Y. S.., Pan M. H.., Shahidi F.Food Chem. 2012. 134:742–748.(7). Steinmann J.., Buer J.., Pietschmann T.., Steinmann E.Br. J. Pharmacol. 2013. 168:1059–1073.(8). Betteridge D. J.Metabolism. 2000. 49:3–8.(9). Yoshikawa T.., Naito Y. J.Japan Med. Ass. 2002. 45:271–276.(10). Gostner J. M.., Becker K.., Ueberall F.., Fuchs D.Food Chem. Toxicol. 2015. 80:72–79.(11). García-Niño W. R.., Zazueta C.Pharmacol. Res. 2015. 97:84–103.(12). de Oliveira C. C.., de Araújo C. V. M.., Ares G.., Granato D.Crit. Rev. Food Sci. Nutr. 2015. 55:1456–1473.(13). Legeay S.., Rodier M.., Fillon L.., Faure S.., Clere N.Nutrients. 2015. 7:5443–5468.(14). Tsai C. F.., Hsu Y. W.., Ting H. C.., Huang C. F.., Yen C. C.Food Chem. 2013. 136:1337–1344.(15). Zhang W. M.., Huang W.Y.., Chen W. X.., Han L.., Zhang H. D.Molecules. 2014. 19:16416–16427.(16). Jeong J. Y.., Jo Y. H.., Kim S. B.., Liu Q.., Lee J. W.., Mo E. J.., Lee K. Y.., Hwang B. Y.., Lee M. K.Bioorg. Med. Chem. Lett. 2015. 25:2269–2274.(17). Jeong J. Y.., Jo Y. H.., Lee K. Y.., Do S. G.., Hwang B. Y.., Lee M. K.Bioorg. Med. Chem. Lett. 2014. 24:2329–2333.(18). Bezerra M. A.., Santelli R. E.., Oliveira E. P.., Villar L. S.., Escaleira L. A.Talanta. 2008. 76:965–977.(19). Ferreira S. L. C.., Bruns R. E.., Ferreira H. S.., Matos G. D.., David J. M.., Brandão G. C.., Da Silva E. G. P.., Portugal L. A.., dos Reis P. S.., Souza A. S.., dos Santos W. N. L.Anal. Chim. Acta. 2007. 597:179–186.(20). Kim S. B.., Jo Y. H.., Liu Q.., Ahn J. H.., Hong I. P.., Han S. M.., Hwang B. Y.., Lee M. K.Molecules. 2015. 20:19764–19774.(21). Lambert J. D.., Elias R. J.Arch. Biochem. Biophys. 2010. 501:65–72.(22). Senthil K. V.., Arulmathi K.., Srividhya R.., Kalaiselvi P.Exp. Gerontol. 2008. 43:176–18.(23). Jovanovic S. V.., Hara Y.., Steenken S.., Simic M. G. J.Am. Chem. Soc. 1995. 117:9881–9888.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidant Activity and Phenolic Content of Different Parts of Lotus and Optimization of Extraction Condition using Response Surface Methodology
- Optimization of the Extraction of Polyphenols and Flavonoids from Argania spinosa Leaves using Response Surface Methodology
- Polyphenols in peanut shells and their antioxidant activity: optimal extraction conditions and the evaluation of antiobesity effects
- A Case of Green Tea-induced Occupational Asthma
- Optimization of Extraction Condition of Hesperidin in Citrus unshiu Peels using Response Surface Methodology