J Clin Neurol.
2017 Apr;13(2):175-180. 10.3988/jcn.2017.13.2.175.
Large-Scale in-House Cell-Based Assay for Evaluating the Serostatus in Patients with Neuromyelitis Optica Spectrum Disorder Based on New Diagnostic Criteria
- Affiliations
-
- 1Department of Neurology, Research Institute and Hospital of National Cancer Center, Goyang, Korea. hojinkim@ncc.re.kr
- 2Division of Translational and Clinical Research II, Research institute, National Cancer Center, Goyang, Korea.
- 3Department of Neurology, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Biochemistry and Molecular Biology, and Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2376019
- DOI: http://doi.org/10.3988/jcn.2017.13.2.175
Abstract
- BACKGROUND AND PURPOSE
The detection of aquaporin 4-IgG (AQP4-IgG) is now a critical diagnostic criterion for neuromyelitis optica spectrum disorder (NMOSD). To evaluate the serostatus of NMOSD patients based on the 2015 new diagnostic criteria using a new in-house cell-based assay (CBA).
METHODS
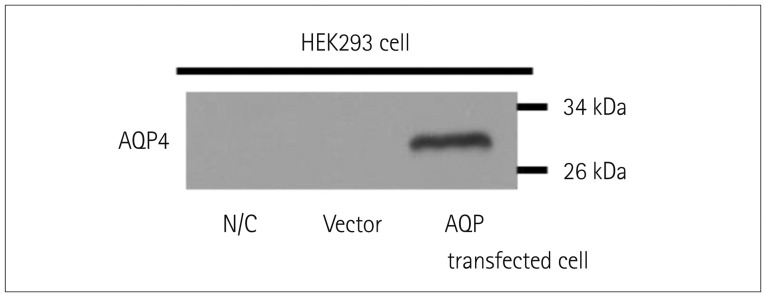
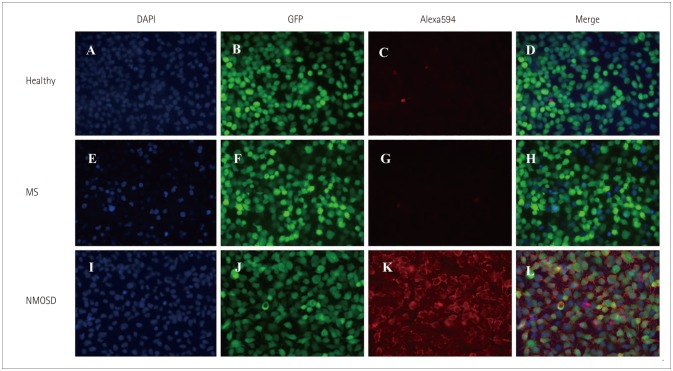
We generated a stable cell line using internal ribosome entry site-containing bicistronic vectors, which allow the simultaneous expression of two proteins (AQP4 and green fluorescent protein) separately from the same RNA transcript. We performed in-house CBA using serum from 386 patients: 178 NMOSD patients diagnosed according to the new diagnostic criteria without AQP4-IgG, 63 high risk NMOSD patients presenting 1 of the 6 core clinical characteristics of NMOSD but not fulfilling dissemination in space, and 145 patients with other neurological diseases, including 66 with multiple sclerosis. The serostatus of 111 definite and high risk NMOSD patients were also tested using a commercial CBA kit with identical serum to evaluate the correlation between the 2 methods. All assays were performed by two independent and blinded investigators.
RESULTS
Our in-house assay yielded a specificity of 100% and sensitivities of 80% (142 of 178) and 76% (48 of 63) when detecting definite- and high risk NMOSD patients, respectively. The comparison with the commercial CBA kit revealed a correlation for 102 of the 111 patients: no correlation was present in 7 patients who were seronegative using the commercial method but seropositive using the in-house method, and in 2 patients who were seropositive using the commercial method but seronegative using the in-house method.
CONCLUSIONS
These results demonstrate that our in-house CBA is a highly specific and sensitive method for detecting AQP4-IgG in NMOSD patients.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Comparison of Neuropathic Pain in Neuromyelitis Optica Spectrum Disorder and Multiple Sclerosis
Jae-Won Hyun, Hyunmin Jang, JaeBin Yu, Na Young Park, Su-Hyun Kim, So-Young Huh, Woojun Kim, Min Su Park, Jeeyoung Oh, Kee Duk Park, Ho Jin Kim
J Clin Neurol. 2020;16(1):124-130. doi: 10.3988/jcn.2020.16.1.124.Investigation of serum biomarkers for neuropathic pain in neuromyelitis optica spectrum disorder: a preliminary study
Jae-Won Hyun, Yeseul Kim, Ho Jin Kim
Ann Clin Neurophysiol. 2021;23(1):46-52. doi: 10.14253/acn.2021.23.1.46.
Reference
-
1. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004; 364:2106–2112. PMID: 15589308.
Article2. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005; 202:473–477. PMID: 16087714.
Article3. Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007; 130:1194–1205. PMID: 17282996.
Article4. Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009; 66:630–643. PMID: 19937948.
Article5. Hinson SR, McKeon A, Lennon VA. Neurological autoimmunity targeting aquaporin-4. Neuroscience. 2010; 168:1009–1018. PMID: 19699271.
Article6. Verkman AS, Phuan PW, Asavapanumas N, Tradtrantip L. Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO. Brain Pathol. 2013; 23:684–695. PMID: 24118484.
Article7. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015; 85:177–189. PMID: 26092914.
Article8. Waters PJ, McKeon A, Leite MI, Rajasekharan S, Lennon VA, Villalobos A, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012; 78:665–671. discussion 669. PMID: 22302543.
Article9. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69:292–302. PMID: 21387374.
Article10. Waters P, Reindl M, Saiz A, Schanda K, Tuller F, Kral V, et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry. 2016; 87:1005–1015. PMID: 27113605.
Article11. Hinson SR, Romero MF, Popescu BF, Lucchinetti CF, Fryer JP, Wolburg H, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci U S A. 2012; 109:1245–1250. PMID: 22128336.
Article12. Kalluri SR, Illes Z, Srivastava R, Cree B, Menge T, Bennett JL, et al. Quantification and functional characterization of antibodies to native aquaporin 4 in neuromyelitis optica. Arch Neurol. 2010; 67:1201–1208. PMID: 20937947.
Article13. Jarius S, Wildemann B. Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol. 2013; 23:661–683. PMID: 24118483.
Article14. Mader S, Lutterotti A, Di Pauli F, Kuenz B, Schanda K, Aboul-Enein F, et al. Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica. PLoS One. 2010; 5:e10455. PMID: 20463974.
Article15. Pisani F, Sparaneo A, Tortorella C, Ruggieri M, Trojano M, Mola MG, et al. Aquaporin-4 autoantibodies in neuromyelitis optica: AQP4 isoform-dependent sensitivity and specificity. PLoS One. 2013; 8:e79185. PMID: 24260168.
Article16. Kitley J, Woodhall M, Leite MI, Palace J, Vincent A, Waters P. Aquaporin-4 antibody isoform binding specificities do not explain clinical variations in NMO. Neurol Neuroimmunol Neuroinflamm. 2015; 2:e121. PMID: 26140280.
Article17. Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. J Biol Chem. 2011; 286:16516–16524. PMID: 21454592.
Article18. Iorio R, Fryer JP, Hinson SR, Fallier-Becker P, Wolburg H, Pittock SJ, et al. Astrocytic autoantibody of neuromyelitis optica (NMO-IgG) binds to aquaporin-4 extracellular loops, monomers, tetramers and high order arrays. J Autoimmun. 2013; 40:21–27. PMID: 22906356.
Article19. Sato DK, Nakashima I, Takahashi T, Misu T, Waters P, Kuroda H, et al. Aquaporin-4 antibody-positive cases beyond current diagnostic criteria for NMO spectrum disorders. Neurology. 2013; 80:2210–2216. PMID: 23677744.
Article20. Marignier R, Bernard-Valnet R, Giraudon P, Collongues N, Papeix C, Zéphir H, et al. Aquaporin-4 antibody-negative neuromyelitis optica: distinct assay sensitivity-dependent entity. Neurology. 2013; 80:2194–2200. PMID: 23658379.
Article21. Rossi A, Ratelade J, Papadopoulos MC, Bennett JL, Verkman AS. Neuromyelitis optica IgG does not alter aquaporin-4 water permeability, plasma membrane M1/M23 isoform content, or supramolecular assembly. Glia. 2012; 60:2027–2039. PMID: 22987455.
Article22. Nicchia GP, Mastrototaro M, Rossi A, Pisani F, Tortorella C, Ruggieri M, et al. Aquaporin-4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies. Glia. 2009; 57:1363–1373. PMID: 19229993.
Article23. Tuller F, Holzer H, Schanda K, Aboulenein-Djamshidian F, Höftberger R, Khalil M, et al. Characterization of the binding pattern of human aquaporin-4 autoantibodies in patients with neuromyelitis optica spectrum disorders. J Neuroinflammation. 2016; 13:176. PMID: 27371173.
Article24. Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future. Anal Bioanal Chem. 2010; 397:3173–3178. PMID: 20549496.
Article25. Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994; 263:802–805. PMID: 8303295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Postpartum Relapse of Neuromyelitis Optica Spectrum Disorder after a Long Period of Spontaneous Remission
- Early relapse after rituximab treatment in a patient with seronegative neuromyelitis optica spectrum disorder: a case report
- Neuromyelitis Optica Spectrum Disorder Presenting with Pseudoathetosis
- Differential Diagnosis between Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder
- Isolated Tongue Paralysis as Presentation of Seropositive Neuromyelitis Optica Spectrum Disorder