Lab Anim Res.
2017 Mar;33(1):24-31. 10.5625/lar.2017.33.1.24.
Effects of coenzyme Qâ‚â‚€ on the antioxidant system in SD rats exposed to lipopolysaccharide-induced toxicity
- Affiliations
-
- 1Department of Animal Science and Biotechnology, and the Regional Animal Research Center, Gyeongnam National University of Science and Technology, Jinju, Korea. isjang@gntech.ac.kr
- 2Department of Clinical Laboratory Science, Dong-Eui Univerisity, Busan, Korea.
- KMID: 2375269
- DOI: http://doi.org/10.5625/lar.2017.33.1.24
Abstract
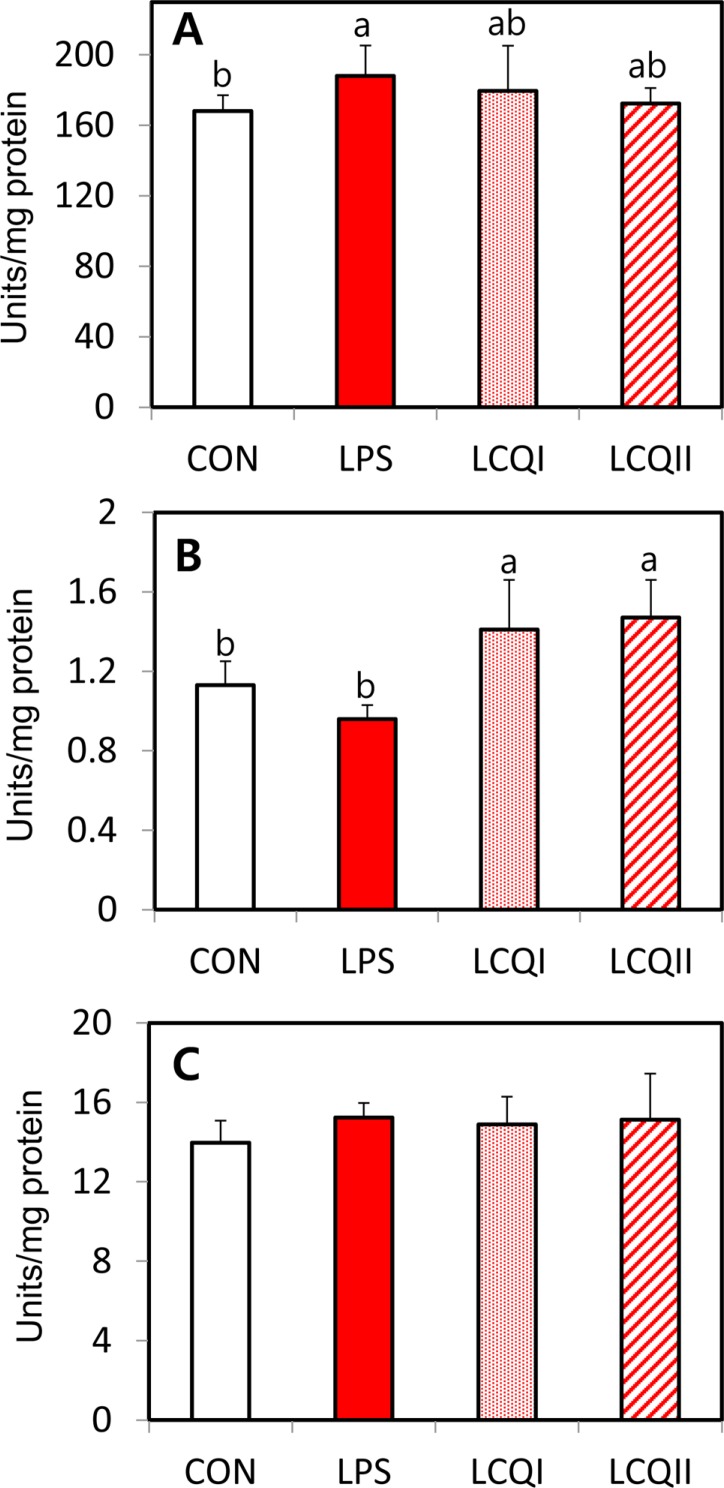
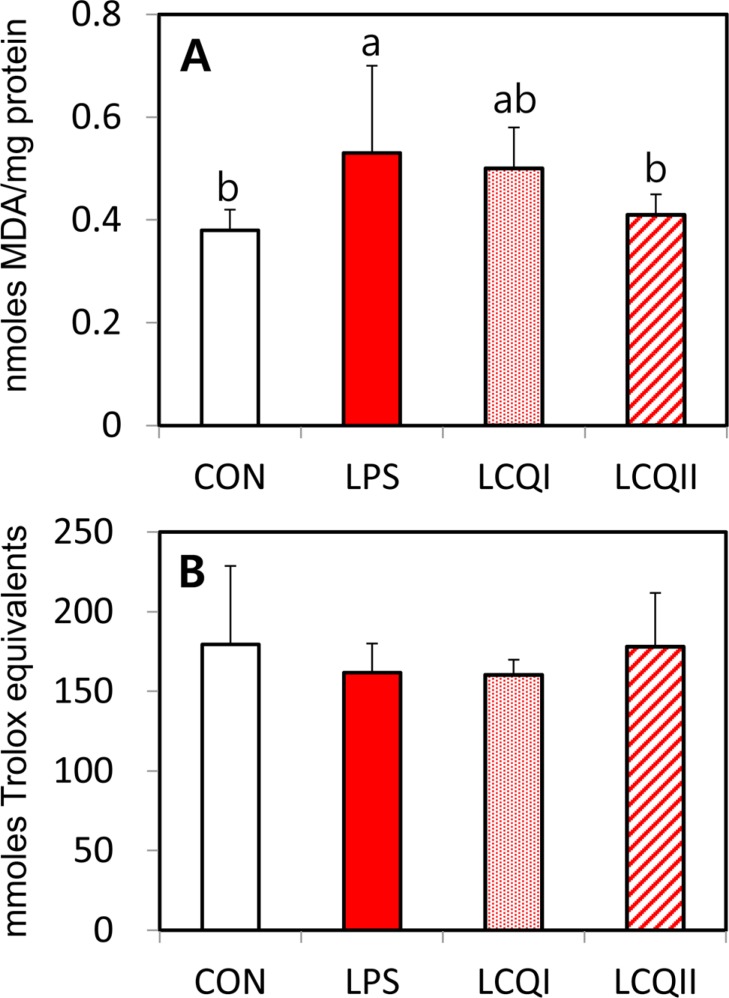
- The study was performed to see the effects of coenzyme Qâ‚â‚€ (CoQâ‚â‚€) on blood biochemical components and hepatic antioxidant system in rats exposed to lipopolysaccharide (LPS)-induced toxicity. A total of 24 rats were allocated to four groups: control (CON), 100 mg/kg BW of LPS (LPS), 100 mg of CoQâ‚â‚€/kg BW with LPS (LCQI) and 300 mg of CoQâ‚â‚€/kg BW with LPS (LCQII). The LPS and LCQI groups showed a significant (P<0.05) increase in the relative spleen weight compared with the CON group without affecting body and liver weights. The blood alanine aminotransferase (ALT) level in the LPS group was significantly (P<0.05) greater than that in the CON group, while supplementation with 100 or 300 mg CoQâ‚â‚€ to rats injected with LPS normalized the ALT level in the CON group. In antioxidant systems, the LPS group showed a significantly (P<0.05) higher mRNA and activity of superoxide dismutase (SOD) than the CON group. The supplementation with CoQâ‚â‚€ to the LPS-treated group normalized the level of SOD, which was comparable to the level of the CON group. Both the mRNA expression and activity of glutathione peroxidase in the LCQI and LCQII groups were higher (P<0.05) than that of the LPS group. However, administration of LPS or CoQâ‚â‚€ unaffected the level of catalase and total antioxidant power. The level of lipid peroxidation in the LCQII group was lower (P<0.05) than that in the LPS group. In conclusion, CoQâ‚â‚€ exerted its favorable effect against liver damage by modulation of antioxidant enzymes in LPS treated rats.
MeSH Terms
Figure
Reference
-
1. Littarru GP. Energy and Defense. Facts and perspectives on coenzyme Q10 in biology and medicine. Roma: Casa Editrice Scientifica Internazionale;1995.2. Quiles JL, Ochoa JJ, Battino M, Gutierrez-Rios P, Nepomuceno EA, Frías ML, Huertas JR, Mataix J. Life-long supplementation with a low dosage of coenzyme Q10 in the rat: effects on antioxidant status and DNA damage. Biofactors. 2005; 25:73–86. PMID: 16873932.3. Acosta MJ, Vazquez Fonseca L, Desbats MA, Cerqua C1, Zordan R, Trevisson E, Salviati L. Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta. 2016; 1857(8):1079–1085. PMID: 27060254.
Article4. Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004; 1660:171–199. PMID: 14757233.
Article5. Littarru GP, Tiano L, Belardinelli R, Watts GF. Coenzyme Q(10), endothelial function, and cardiovascular disease. Biofactors. 2011; 37(5):366–373. PMID: 21674640.
Article6. Yang YK, Wang LP, Chen L, Yao XP, Yang KQ, Gao LG, Zhou XL. Coenzyme Q10 treatment of cardiovascular disorders of ageing including heart failure, hypertension and endothelial dysfunction. Clin Chim Acta. 2015; 450:83–89. PMID: 26254995.7. Kamzalov S, Sumien N, Forster MJ, Sohal RS. Coenzyme Q intake elevates the mitochondrial and tissue levels of Coenzyme Q and alpha-tocopherol in young mice. J Nutr. 2003; 133(10):3175–3180. PMID: 14519806.8. Bullón P, Román-Malo L, Marín-Aguilar F, Alvarez-Suarez JM, Giampieri F, Battino M, Cordero MD. Lipophilic antioxidants prevent lipopolysaccharide-induced mitochondrial dysfunction through mitochondrial biogenesis improvement. Pharmacol Res. 2015; 91:1–8. PMID: 25447593.
Article9. James AM, Smith RA, Murphy MP. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch Biochem Biophys. 2004; 423(1):47–56. PMID: 14989264.
Article10. Niklowitz P, Menke T, Andler W, Okun JG. Simultaneous analysis of coenzyme Q10 in plasma, erythrocytes and platelets: comparison of the antioxidant level in blood cells and their environment in healthy children and after oral supplementation in adults. Clin Chim Acta. 2004; 342:219–226. PMID: 15026284.11. Shekelle P, Morton S, Hardy ML. Effect of supplemental antioxidants vitamin C, vitamin E, and coenzyme Q10 for the prevention and treatment of cardiovascular disease. Evid Rep Technol Assess (Summ). 2003; (83):1–3.12. Rötig A, Appelkvist EL, Geromel V, Chretien D, Kadhom N, Edery P, Lebideau M, Dallner G, Munnich A, Ernster L, Rustin P. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000; 356(9227):391–395. PMID: 10972372.13. Novoselova EG, Lunin SM, Novoselova TV, Khrenov MO, Glushkova OV, Avkhacheva NV, Safronova VG, Fesenko EE. Naturally occurring antioxidant nutrients reduce inflammatory response in mice. Eur J Pharmacol. 2009; 615:234–240. PMID: 19463810.
Article14. Maruyama H, Furukawa K, Onda M. Effect of coenzyme Q10 on endotoxin induced hepatocyte injury modulation of endotoxin-activated polymorphonuclear neutrophils. Nihon Ika Daigaku Zasshi. 1995; 62(3):271–282. PMID: 7615699.15. Frei B, Kim MC, Ames BN. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc Natl Acad Sci U S A. 1990; 87(12):4879–4883. PMID: 2352956.
Article16. Sohal RS, Kamzalov S, Sumien N, Ferguson M, Rebrin I, Heinrich KR, Forster MJ. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic Biol Med. 2006; 40(3):480–487. PMID: 16443163.17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4):402–408. PMID: 11846609.18. Kupfer D, Levin E. Monooxygenase drug metabolizing activity in CaCl2-aggregated hepatic microsomes from rat liver. Biochem Biophys Res Commun. 1972; 47(3):611–618. PMID: 4402636.19. Tappel AL. Glutathione peroxidase and hydroperoxides. Methods in Enzymol. 1978; 52:506–513. PMID: 672654.20. Melega S, Canistro D, De Nicola GR, Lazzeri L, Sapone A, Paolini M. Protective effect of Tuscan black cabbage sprout extract against serum lipid increase and perturbations of liver antioxidant and detoxifying enzymes in rats fed a high-fat diet. Br J Nutr. 2013; 110(6):988–997. PMID: 23433361.
Article21. Bidlack WR, Tappel AL. Damage to microsomal membrane by lipid peroxidation. Lipids. 1973; 8(4):177–182. PMID: 4348615.
Article22. Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, Celle G, Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999; 44(6):1249–1253. PMID: 10389705.23. Li Q, Liu Y, Che Z, Zhu H, Meng G, Hou Y, Ding B, Yin Y, Chen F. Dietary L-arginine supplementation alleviates liver injury caused by Escherichia coli LPS in weaned pigs. Innate Immun. 2012; 18(6):804–814. PMID: 22441699.
Article24. El-Tanbouly DM, Abdelsalam RM, Attia AS, Abdel-Aziz MT. Pretreatment with magnesium ameliorates lipopolysaccharide-induced liver injury in mice. Pharmacol Rep. 2015; 67(5):914–920. PMID: 26398385.
Article25. Ali SA, Faddah L, Abdel-Baky A, Bayoumi A. Protective effect of L-carnitine and coenzyme Q10 on CCl4-induced liver injury in rats. Sci Pharm. 2010; 78(4):881–896. PMID: 21179323.26. Fouad AA, Jresat I. Hepatoprotective effect of coenzyme Q10 in rats with acetaminophen toxicity. Environ Toxicol Pharmacol. 2012; 33(2):158–167. PMID: 22222558.27. Bian K, Murad F. Diversity of endotoxin-induced nitrotyrosine formation in macrophage-endothelium-rich organs. Free Radic Biol Med. 2001; 31(4):421–429. PMID: 11498275.
Article28. Fridovich I. Superoxide dismutase. Adv Enzymol Relat Areas Mol Biol. 1974; 41:35–40. PMID: 4371571.
Article29. Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000; 153:83–104. PMID: 11090949.
Article30. Chauhan DP, Gupta PH, Nampoothiri MR, Singhal PC, Chugh KS, Nair CR. Determination of erythrocyte superoxide dismutase, catalase, glucose-6-phosphate dehydrogenase, reduced glutathione and malonyldialdehyde in uremia. Clin Chim Acta. 1982; 123:153–159. PMID: 6749335.
Article31. Nishimura N, Ito Y, Adachi T, Hirano K, Sugiura M, Sawaki S. Enzyme immunoassay for cuprozinc-superoxide dismutase in serum and urine. J Pharmacobiodyn. 1982; 5(6):394–402. PMID: 6750078.
Article32. Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011; 31(5):379–446. PMID: 22142165.
Article33. Chou ST, Peng HY, Hsu JC, Lin CC, Shih Y. Achillea millefolium L. essential oil inhibits LPS-induced oxidative stress and nitric oxide production in RAW 264.7 Macrophages. Int J Mol Sci. 2013; 14(7):12978–12993. PMID: 23797659.
Article34. Spolarics Z. Endotoxin stimulates gene expression of ROS-eliminating pathways in rat hepatic endothelial and Kupffer cells. Am J Physiol. 1996; 270:G660–G666. PMID: 8928796.
Article35. Sena CM, Nunes E, Gomes A, Santos MS, Proença T, Martins MI, Seiça RM. Supplementation of coenzyme Q10 and alpha-tocopherol lowers glycated hemoglobin level and lipid peroxidation in pancreas of diabetic rats. Nutr Res. 2008; 28(2):113–121. PMID: 19083397.36. Ahmadvand H, Tavafi M, Khosrowbeygi A. Amelioration of altered antioxidant enzymes activity and glomerulosclerosis by coenzyme Q10 in alloxan-induced diabetic rats. J Diabetes Complications. 2012; 26(6):476–482. PMID: 22795334.37. Lee CK, Pugh TD, Klopp RG, Edwards J, Allison DB, Weindruch R, Prolla TA. The impact of alpha-lipoic acid, coenzyme Q10 and caloric restriction on life span and gene expression patterns in mice. Free Radic Biol Med. 2004; 36(8):1043–1057. PMID: 15059645.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of coenzyme Qâ‚â‚€ supplement on blood lipid indices and hepatic antioxidant defense system in SD rats fed a high cholesterol diet
- An experimental study on the effect of maltol against oxygen toxicity
- An Experimental Study on the Efficacy of Vitamin E against Oxygen Toxicity
- Increase in rat plasma antioxidant activity after E. coli lipopolysaccharide administration
- Quinic Acid Alleviates Behavior Impairment by Reducing Neuroinflammation and MAPK Activation in LPS-Treated Mice