J Korean Med Sci.
2017 May;32(5):737-743. 10.3346/jkms.2017.32.5.737.
Opsonophagocytic Antibodies to Serotype Ia, Ib, and III Group B Streptococcus among Korean Infants and in Intravenous Immunoglobulin Products
- Affiliations
-
- 1Center for Vaccine Evaluation and Study, Medical Research Institute, Ewha Womans University School of Medicine, Seoul, Korea. kaykim@ewha.ac.kr
- 2Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Graduate School of Medicine, Gachon University, Incheon, Korea.
- 4Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- 5Biotechnology Division, Korea Atomic Energy Research Institute, Jeongeup, Korea.
- KMID: 2375072
- DOI: http://doi.org/10.3346/jkms.2017.32.5.737
Abstract
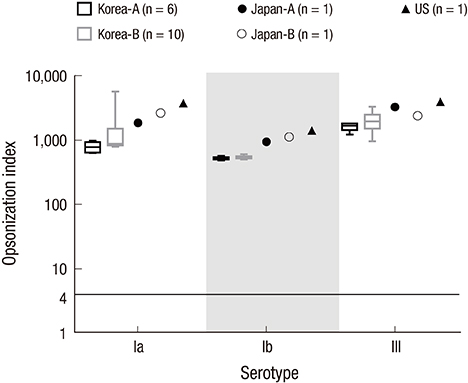
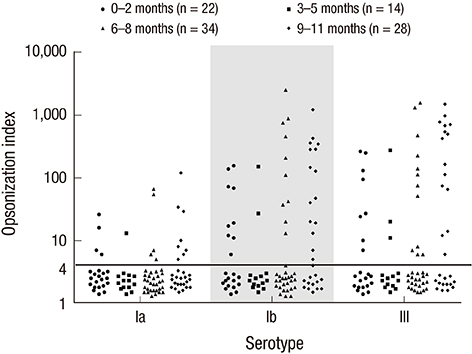
- Group B streptococcus (GBS) infection is a leading cause of sepsis and meningitis among infants, and is associated with high rates of morbidity and mortality in many countries. Protection against GBS typically involves antibody-mediated opsonization by phagocytes and complement components. The present study evaluated serotype-specific functional antibodies to GBS among Korean infants and in intravenous immunoglobulin (IVIG) products. An opsonophagocytic killing assay (OPA) was used to calculate the opsonization indices (OIs) of functional antibodies to serotypes Ia, Ib, and III in 19 IVIG products from 5 international manufacturers and among 98 Korean infants (age: 0-11 months). The GBS Ia, Ib, and III serotypes were selected because they are included in a trivalent GBS vaccine formulation that is being developed. The OI values for the IVIG products were 635-5,706 (serotype Ia), 488-1,421 (serotype Ib), and 962-3,315 (serotype III), and none of the IVIG lots exhibited undetectable OI values (< 4). The geometric mean OI values were similar for all 3 serotypes when we compared the Korean manufacturers. The seropositive rate among infants was significantly lower for serotype Ia (18.4%), compared to serotype Ib and serotype III (both, 38.8%). Infant age of ≥ 3 months was positively correlated with the seropositive rates for each serotype. Therefore, only a limited proportion of infants exhibited protective immunity against serotype Ia, Ib, and III GBS infections. IVIG products that exhibit high antibody titers may be a useful therapeutic or preventive measure for infants. Further studies are needed to evaluate additional serotypes and age groups.
Keyword
MeSH Terms
Figure
Reference
-
1. Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008; 299:2056–2065.2. Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine. 2013; 31:Suppl 4. D7–12.3. Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976; 294:753–756.4. Boyer KM, Papierniak CK, Gadzala CA, Parvin JD, Gotoff SP. Transplacental passage of IgG antibody to group B streptococcus serotype Ia. J Pediatr. 1984; 104:618–620.5. Gotoff SP, Papierniak CK, Klegerman ME, Boyer KM. Quantitation of IgG antibody to the type-specific polysaccharide of group B streptococcus type 1b in pregnant women and infected infants. J Pediatr. 1984; 105:628–630.6. Baker CJ, Rench MA, McInnes P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine. 2003; 21:3468–3472.7. Edwards MS, Lane HJ, Hillier SL, Rench MA, Baker CJ. Persistence of functional antibodies to group B streptococcal capsular polysaccharides following immunization with glycoconjugate vaccines. Vaccine. 2012; 30:4123–4126.8. Dangor Z, Kwatra G, Izu A, Lala SG, Madhi SA. Review on the association of Group B streptococcus capsular antibody and protection against invasive disease in infants. Expert Rev Vaccines. 2015; 14:135–149.9. Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006; 13:165–169.10. Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006; 13:1004–1009.11. Heyderman RS, Madhi SA, French N, Cutland C, Ngwira B, Kayambo D, Mboizi R, Koen A, Jose L, Olugbosi M, et al. Group B streptococcus vaccination in pregnant women with or without HIV in Africa: a non-randomised phase 2, open-label, multicentre trial. Lancet Infect Dis. 2016; 16:546–555.12. Madhi SA, Dangor Z, Heath PT, Schrag S, Izu A, Sobanjo-Ter Meulen A, Dull PM. Considerations for a phase-III trial to evaluate a group B streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine. 2013; 31:Suppl 4. D52–7.13. Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox Sang. 2010; 98:12–28.14. Mikolajczyk MG, Concepcion NF, Wang T, Frazier D, Golding B, Frasch CE, Scott DE. Characterization of antibodies to capsular polysaccharide antigens of Haemophilus influenzae type b and Streptococcus pneumoniae in human immune globulin intravenous preparations. Clin Diagn Lab Immunol. 2004; 11:1158–1164.15. Adam E, Church JA. Antibody levels to Bordetella pertussis and Neisseria meningitidis in immunodeficient patients receiving immunoglobulin replacement therapy. J Clin Immunol. 2015; 35:213–217.16. Takahashi Y, Ishiwada N, Hishiki H, Tanaka J, Akeda Y, Shimojo N, Oishi K, Kohno Y. IgG levels against 13-valent pneumococcal conjugate vaccine serotypes in non pneumococcal conjugate vaccine immunized healthy Japanese and intravenous immunoglobulin preparations. J Infect Chemother. 2014; 20:794–798.17. Schuchat A. Group B streptococcus. Lancet. 1999; 353:51–56.18. Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA. Natural acquired humoral immunity against serotype-specific group B streptococcus rectovaginal colonization acquisition in pregnant women. Clin Microbiol Infect. 2015; 21:568.e13–568.e21.19. Edwards MS, Munoz FM, Baker CJ. Antibodies to type III group B streptococcal polysaccharide in breast milk. Pediatr Infect Dis J. 2004; 23:961–963.20. Baker CJ, Edwards MS, Kasper DL. Role of antibody to native type III polysaccharide of group B streptococcus in infant infection. Pediatrics. 1981; 68:544–549.21. Melin P, Efstratiou A. Group B streptococcal epidemiology and vaccine needs in developed countries. Vaccine. 2013; 31:Suppl 4. D31–42.22. Uh Y, Choi SJ, Jang IH, Lee KS, Cho HM, Kwon O, Yoon KJ. Colonization rate, serotypes, and distributions of macrolide-lincosamide-streptograminB resistant types of group B streptococci in pregnant women. Korean J Clin Microbiol. 2009; 12:174–179.23. Oh CE, Jang HO, Kim NH, Lee J, Choi EH, Lee HJ. Molecular serotyping of group B streptococcus isolated from the pregnant women by polymerase chain reaction and sequence analysis. Korean J Pediatr Infect Dis. 2009; 16:47–53.24. Hong JS, Choi CW, Park KU, Kim SN, Lee HJ, Lee HR, Choi EH, Park KH, Suh CS, Kim BI, et al. Genital group B streptococcus carrier rate and serotype distribution in Korean pregnant women: implications for group B streptococcal disease in Korean neonates. J Perinat Med. 2010; 38:373–377.25. Fischer GW. Immunoglobulin therapy for neonatal sepsis: an overview of animal and clinical studies. J Clin Immunol. 1990; 10:40S–6S.26. Ohlsson A, Lacy J. Intravenous immunoglobulin for suspected or subsequently proven infection in neonates. Cochrane Database Syst Rev. 2010; CD001239.27. INIS Collaborative Group. Brocklehurst P, Farrell B, King A, Juszczak E, Darlow B, Haque K, Salt A, Stenson B, Tarnow-Mordi W. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med. 2011; 365:1201–1211.28. Baker CJ, Noya FJ. Potential use of intravenous immune globulin for group B streptococcal infection. Rev Infect Dis. 1990; 12:Suppl 4. S476–S482.29. Baker CJ, Rench MA, Noya FJ, Garcia-Prats JA. Role of intravenous immunoglobulin in prevention of late-onset infection in low-birth-weight neonates. The Neonatal IVIG Study Group. Rev Infect Dis. 1990; 12:Suppl 4. S463–S468.30. Madzivhandila M, Adrian PV, Cutland CL, Kuwanda L, Schrag SJ, Madhi SA. Serotype distribution and invasive potential of group B streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS One. 2011; 6:e17861.31. Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, Heath PT. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012; 379:547–556.32. Cho HK, Park IH, Burton RL, Kim KH. Impact of IgM antibodies on cross-protection against pneumococcal serogroups 6 and 19 after immunization with 7-valent pneumococcal conjugate vaccine in children. J Korean Med Sci. 2016; 31:950–956.33. Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun. 2011; 79:314–320.34. Lee H, Nahm MH, Burton R, Kim KH. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin Vaccine Immunol. 2009; 16:376–381.35. Hall MA, Edwards MS, Baker CJ. Complement and antibody participation in opsonophagocytosis of type IV and V group B streptococci. Infect Immun. 1992; 60:5030–5035.36. Fleck RA, Romero-Steiner S, Nahm MH. Use of HL-60 cell line to measure opsonic capacity of pneumococcal antibodies. Clin Diagn Lab Immunol. 2005; 12:19–27.37. Clarke ML, Burton RL, Hill AN, Litorja M, Nahm MH, Hwang J. Low-cost, high-throughput, automated counting of bacterial colonies. Cytometry A. 2010; 77:790–797.38. Lin FY, Weisman LE, Azimi PH, Philips JB 3rd, Clark P, Regan J, Rhoads GG, Frasch CE, Gray BM, Troendle J, et al. Level of maternal IgG anti-group B streptococcus type III antibody correlated with protection of neonates against early-onset disease caused by this pathogen. J Infect Dis. 2004; 190:928–934.39. Baker CJ, Carey VJ, Rench MA, Edwards MS, Hillier SL, Kasper DL, Platt R. Maternal antibody at delivery protects neonates from early onset group B streptococcal disease. J Infect Dis. 2014; 209:781–788.40. Dangor Z, Kwatra G, Izu A, Adrian P, Cutland CL, Velaphi S, Ballot D, Reubenson G, Zell ER, Lala SG, et al. Correlates of protection of serotype-specific capsular antibody and invasive Group B streptococcus disease in South African infants. Vaccine. 2015; 33:6793–6799.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Seroprevalence of Opsonophagocytic Antibodies against Serotype Ia, Ib, II, III, and V Group B Streptococcus among Korean Population
- Opsonophagocytic Antibodies of Intravenous Immunoglobulin Preparations against Seven Streptococcus agalactiae Serotypes
- Molecular Serotyping of Group B Streptococcus Isolated from the Pregnant Women by Polymerase Chain Reaction and Sequence Analysis
- Evaluation of antibody responses to pneumococcal vaccines with ELISA and opsonophagocytic assay
- Functional Immunity to Cross-Reactive Serotype 6A Induced by Serotype 6B in Pneumococcal Polysaccharide Vaccine