Yonsei Med J.
2016 Sep;57(5):1115-1123. 10.3349/ymj.2016.57.5.1115.
Blood Neutrophil-to-Lymphocyte Ratio Predicts Tumor Recurrence in Patients with Hepatocellular Carcinoma within Milan Criteria after Hepatectomy
- Affiliations
-
- 1Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Ajou University School of Medicine, Suwon, Korea. wanghj@ajou.ac.kr
- 2Department of Surgery, International St. Mary's Hospital, Incheon, Korea.
- 3Department of Surgery, Yanji City Hospital, Jilin, China.
- KMID: 2374155
- DOI: http://doi.org/10.3349/ymj.2016.57.5.1115
Abstract
- PURPOSE
The systemic inflammation biomarker, Neutrophil-to-Lymphocyte Ratio (NLR), has been reported as one of the adverse prognostic factors for hepatocellular carcinoma (HCC) patient. The purpose of this study was to evaluate whether NLR could predict the risk of recurrence and death for the HCC patient, according to Milan criteria after hepatectomy.
MATERIALS AND METHODS
Retrospective analysis was performed on a database of HCC patients who underwent hepatectomy between March 2001 and December 2011. The cutoff value of NLR was decided by receiver operating characteristic (ROC) curve analysis. Univariate and multivariate regression analyses were performed to identify predictive factors of recurrence and death.
RESULTS
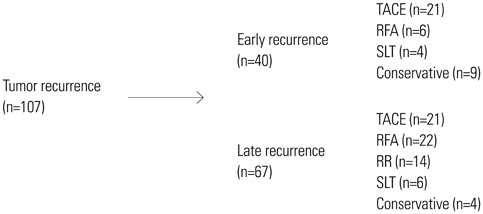
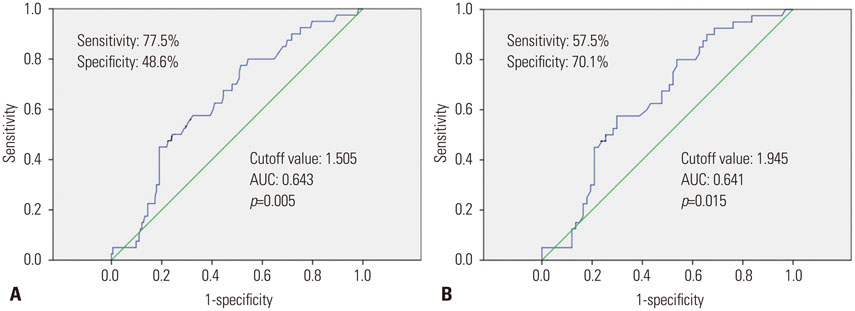
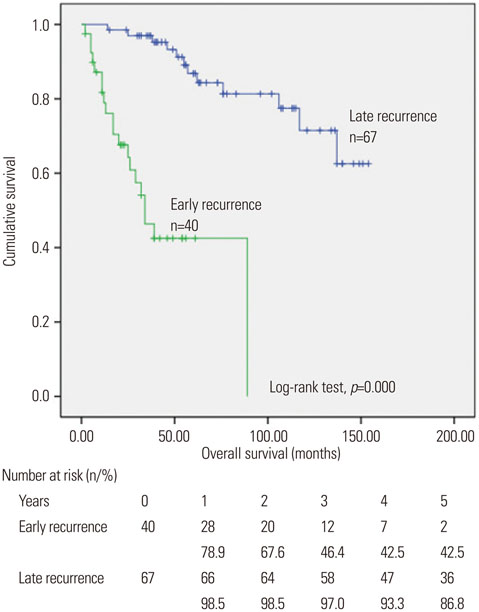
A total of 213 patients were included in the present study. The median follow-up period was 48 months. One hundred and seven patients were experienced tumor recurrence; forty of them recurred within 12 months (early recurrence). NLR ≥1.505, albumin ≤3.75 g/dL, microvascular invasion and high grade of cirrhosis were found to be independent factors for adverse recurrence-free survival in multivariate regression analysis. And NLR ≥1.945 was also found as a prognosis factor for early recurrence by univariate regression analysis.
CONCLUSION
Elevated preoperative NLR can be easily obtained and reliable biomarker for assessing the tumor recurrence and early recurrence of Milan criteria HCC after the initial hepatectomy.
MeSH Terms
-
Adult
Aged
Biomarkers
Biomarkers, Tumor/blood
Carcinoma, Hepatocellular/*surgery
Disease-Free Survival
Female
Follow-Up Studies
Hepatectomy
Humans
Liver Neoplasms/*surgery
Lymphocyte Count
*Lymphocytes
Male
Middle Aged
Neoplasm Recurrence, Local/*blood
*Neutrophils
ROC Curve
Retrospective Studies
Biomarkers
Biomarkers, Tumor
Figure
Reference
-
1. Song IH, Kim KS. Current status of liver diseases in Korea: hepatocellular carcinoma. Korean J Hepatol. 2009; 15:15 Suppl 6. S50–S59.
Article2. Park KW, Park JW, Choi JI, Kim TH, Kim SH, Park HS, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008; 23:467–473.
Article3. Jung SH, Kim BH, Joung YH, Han YS, Lee BH, Dong SH, et al. [Clinical features of hepatocellular carcinoma in the 1990s]. Korean J Gastroenterol. 2003; 42:322–329.4. Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003; 238:703–710.
Article5. Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001; 102:5–14.6. Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008; 32:1757–1762.
Article7. Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013; 258:301–305.
Article8. Liao W, Zhang J, Zhu Q, Qin L, Yao W, Lei B, et al. Preoperative neutrophil-to-lymphocyte ratio as a new prognostic marker in hepatocellular carcinoma after curative resection. Transl Oncol. 2014; 7:248–255.
Article9. Huang ZL, Luo J, Chen MS, Li JQ, Shi M. Blood neutrophil-tolymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2011; 22:702–709.
Article10. Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012; 27:553–561.
Article11. Halazun KJ, Hardy MA, Rana AA, Woodland DC 4th, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009; 250:141–151.
Article12. Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013; 58:58–64.
Article13. Harimoto N, Shirabe K, Nakagawara H, Toshima T, Yamashita Y, Ikegami T, et al. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/lymphocyte ratio. Transplantation. 2013; 96:1008–1012.
Article14. Tajiri K, Kawai K, Minemura M, Yasumura S, Hosokawa A, Kawabe H, et al. Neutrophil/lymphocyte ratio as a prognostic indicator of hepatic arterial infusion chemotherapy with arterial cisplatin plus continuous 5-fluorouracil. Hepatol Res. 2015; 45:755–763.
Article15. Zheng YB, Zhao W, Liu B, Lu LG, He X, Huang JW, et al. The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac J Cancer Prev. 2013; 14:5527–5531.
Article16. Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994; 19:1513–1520.
Article17. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954; 7:462–503.
Article18. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet. 2001; 357:539–545.
Article19. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–899.
Article20. Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013; 2013:187204.
Article21. Hotchkiss KA, Ashton AW, Klein RS, Lenzi ML, Zhu GH, Schwartz EL. Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Res. 2003; 63:527–533.22. Marconi C, Bianchini F, Mannini A, Mugnai G, Ruggieri S, Calorini L. Tumoral and macrophage uPAR and MMP-9 contribute to the invasiveness of B16 murine melanoma cells. Clin Exp Metastasis. 2008; 25:225–231.
Article23. Peng SH, Deng H, Yang JF, Xie PP, Li C, Li H, et al. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol. 2005; 11:6521–6524.
Article24. Borish L, Rosenbaum R, Albury L, Clark S. Activation of neutrophils by recombinant interleukin 6. Cell Immunol. 1989; 121:280–289.
Article25. Maniecki MB, Etzerodt A, Ulhøi BP, Steiniche T, Borre M, Dyrskjøt L, et al. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int J Cancer. 2012; 131:2320–2331.
Article26. Varney ML, Olsen KJ, Mosley RL, Bucana CD, Talmadge JE, Singh RK. Monocyte/macrophage recruitment, activation and differentiation modulate interleukin-8 production: a paracrine role of tumor-associated macrophages in tumor angiogenesis. In Vivo. 2002; 16:471–477.27. Zhao Q, Xiao X, Wu Y, Wei Y, Zhu LY, Zhou J, et al. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol. 2011; 41:2314–2322.
Article28. Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010; 51:154–164.
Article29. Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, Wang J, et al. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol. 2010; 185:1544–1549.
Article30. Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008; 15:1375–1382.
Article31. Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000; 89:500–507.
Article32. Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996; 83:1219–1222.
Article33. Cruz NG, Sousa LP, Sousa MO, Pietrani NT, Fernandes AP, Gomes KB. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2013; 99:85–92.
Article34. Shau WY, Shao YY, Yeh YC, Lin ZZ, Kuo R, Hsu CH, et al. Diabetes mellitus is associated with increased mortality in patients receiving curative therapy for hepatocellular carcinoma. Oncologist. 2012; 17:856–862.
Article35. Wang YY, Huang S, Zhong JH, Ke Y, Guo Z, Liu JQ, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. PLoS One. 2014; 9:e113858.
Article36. Poon RT, Fan ST, Wong J. Does diabetes mellitus influence the perioperative outcome or long term prognosis after resection of hepatocellular carcinoma. Am J Gastroenterol. 2002; 97:1480–1488.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Results of Hepatectomy as First Treatment for Hepatocellular Carcinoma within the Milan Criteria with Preserved Liver Function
- Preoperative blood neutrophil count predicts survival in hepatocellular carcinoma patients with living donor liver transplantation
- Effects of Partial Hepatectomy for Hepatocellular Carcinoma Meeting Milan Criteria Combined with Compensated Liver Cirrhosis
- The risk factors of early recurrence after hepatectomy in hepatocellular carcinoma
- Risk of posttransplantation recurrence in hepatocellular carcinoma patients within the Milan criteria: importance for evaluating the recurrence potential