Yonsei Med J.
2016 Sep;57(5):1070-1078. 10.3349/ymj.2016.57.5.1070.
Survival Outcomes of Concurrent Treatment with Docetaxel and Androgen Deprivation Therapy in Metastatic Castration-Resistant Prostate Cancer
- Affiliations
-
- 1Department of Urology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. chung646@yuhs.ac
- KMID: 2374150
- DOI: http://doi.org/10.3349/ymj.2016.57.5.1070
Abstract
- PURPOSE
Docetaxel-based chemotherapy (DTX) improves overall survival (OS) of men with metastatic castration-resistant prostate cancer (mCRPC). Considering the potential existence of androgen receptors that remain active at this stage, we aimed to assess the impact of the combined use of androgen deprivation therapy (ADT) with DTX for mCRPC.
MATERIALS AND METHODS
We performed a single-institutional retrospective analysis of patients with mCRPC who received either DTX alone (DTX group, n=21) or concurrent DTX and ADT (DTX+ADT group, n=26) between August 2006 and February 2014. All patients received DTX doses of 75 mg/m2 every three weeks for at least three cycles. In the DTX+ADT group, all patients used luteinizing hormone releasing hormone agonist continuously as a concurrent ADT.
RESULTS
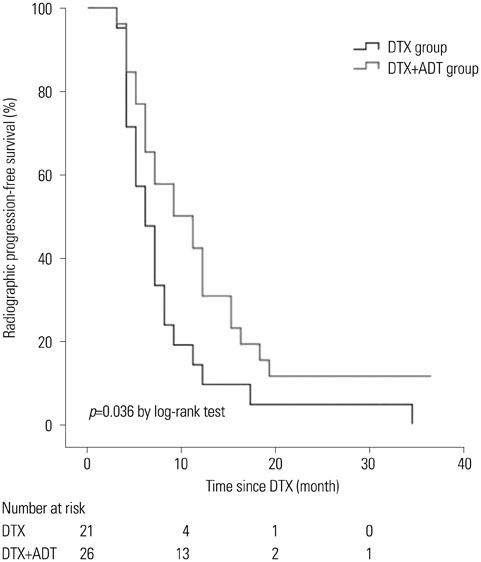
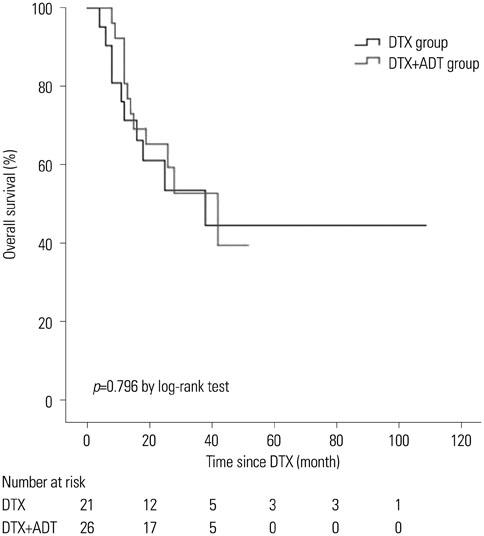
The median follow-up period was 24.0 months (interquartile range 12.0-37.0) for the entire cohort. The median radiographic progression-free survival (rPFS) was 9.0 months and 6.0 months in the DTX+ADT and DTX groups, respectively (log-rank p=0.036). On multivariable Cox regression analysis, concurrent administration of ADT was the only significant predictor of rPFS [hazard ratio (HR)=0.525, 95% confidence intervals (CI) 0.284-0.970, p=0.040]. The median OS was 42.0 and 38.0 months in the DTX+ADT and DTX groups, respectively (log-rank p=0.796). On multivariable analysis, hemoglobin level at the time of DTX initiation was associated with OS (HR=0.532, 95% CI 0.381-0.744, p<0.001).
CONCLUSION
In chemotherapy-naive patients with mCRPC, the combined use of ADT with DTX improved rPFS. Our result suggests that the concurrent administration of ADT and DTX is superior to DTX alone.
Keyword
MeSH Terms
-
Adenocarcinoma/blood/*drug therapy/secondary
Aged
Antineoplastic Combined Chemotherapy Protocols/*therapeutic use
Disease-Free Survival
Gonadotropin-Releasing Hormone/administration & dosage/agonists
Hemoglobins/metabolism
Humans
Male
Middle Aged
Prostatic Neoplasms, Castration-Resistant/blood/*drug therapy/pathology
Retrospective Studies
Survival Rate
Taxoids/administration & dosage
Gonadotropin-Releasing Hormone
Hemoglobins
Taxoids
Figure
Cited by 1 articles
-
Efficacy of Androgen Deprivation Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Docetaxel-Based Chemotherapy
Kyungchan Min, Jae-Wook Chung, Yun-Sok Ha, Jun Nyung Lee, Bum Soo Kim, Hyun Tae Kim, Tae-Hwan Kim, Eun Sang Yoo, Tae Gyun Kwon, Sung Kwang Chung, Masatoshi Tanaka, Shin Egawa, Takahiro Kimura, Seock Hwan Choi
World J Mens Health. 2020;38(2):226-235. doi: 10.5534/wjmh.190029.
Reference
-
1. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon (France): International Agency for Research on Cancer;c2013. updated 2014. cited 2015 Aug 4. Available at: http://globocan.iarc.fr.2. Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D'Amico AV, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014; 12:686–718.
Article3. Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006; 175:27–34.
Article4. Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005; 23:2918–2925.
Article5. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012; 367:1187–1197.
Article6. Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009; 6:76–85.
Article7. Donkena KV, Yuan H, Young CY. Recent advances in understanding hormonal therapy resistant prostate cancer. Curr Cancer Drug Targets. 2010; 10:402–410.
Article8. Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010; 17:Suppl 2. S72–S79.
Article9. Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009; 15:3251–3255.
Article10. Sharifi N, Dahut WL, Figg WD. The genetics of castration-resistant prostate cancer: what can the germline tell us? Clin Cancer Res. 2008; 14:4691–4693.
Article11. Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002; 20:3001–3015.
Article12. Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004; 10:33–39.
Article13. Gregory CW, Johnson RT Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001; 61:2892–2898.14. Buchanan G, Yang M, Harris JM, Nahm HS, Han G, Moore N, et al. Mutations at the boundary of the hinge and ligand binding domain of the androgen receptor confer increased transactivation function. Mol Endocrinol. 2001; 15:46–56.
Article15. Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009; 27:3452–3458.
Article16. Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007; 99:1516–1524.
Article17. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005; 352:154–164.
Article18. Strum SB, McDermed JE, Scholz MC, Johnson H, Tisman G. Anaemia associated with androgen deprivation in patients with prostate cancer receiving combined hormone blockade. Br J Urol. 1997; 79:933–941.
Article19. Taylor CD, Elson P, Trump DL. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993; 11:2167–2172.
Article20. Hussain M, Wolf M, Marshall E, Crawford ED, Eisenberger M. Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: a Southwest Oncology Group report. J Clin Oncol. 1994; 12:1868–1875.
Article21. Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011; 59:572–583.
Article22. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. AJCC cancer staging manual. 7th ed. New York: Springer;2010.23. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [Internet]. Bethesda (MD): National Institutes of Health, National Cancer Institute (US);c2009. updated 2010 Jun 14. cited 2015 Aug 4. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.24. Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999; 17:3461–3467.
Article25. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008; 26:1148–1159.
Article26. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.
Article27. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351:1502–1512.
Article28. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004; 351:1513–1520.
Article29. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364:1995–2005.
Article30. Lee JL, Eun Kim J, Ahn JH, Lee DH, Lee J, Kim CS, et al. Role of androgen deprivation treatment in patients with castration-resistant prostate cancer, receiving docetaxel-based chemotherapy. Am J Clin Oncol. 2011; 34:140–144.
Article31. Sargent D. General and statistical hierarchy of appropriate biologic endpoints. Oncology (Williston Park). 2006; 20:6 Suppl 5. 5–9.32. Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009; 101:1642–1649.
Article33. EMA. Guideline on the evaluation of anticancer medicinal products in man (CHMP/205/95/Rev.4) [Internet]. London (UK): European Medicines Agency;2012. cited 2015 Aug 4. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/01/WC500137128.pdf.34. Sonpavde G, Pond GR, Armstrong AJ, Galsky MD, Leopold L, Wood BA, et al. Radiographic progression by Prostate Cancer Working Group (PCWG)-2 criteria as an intermediate endpoint for drug development in metastatic castration-resistant prostate cancer. BJU Int. 2014; 114:E25–E31.
Article35. Koo KC, Park SU, Kim KH, Rha KH, Hong SJ, Yang SC, et al. Prognostic impacts of metastatic site and pain on progression to castrate resistance and mortality in patients with metastatic prostate cancer. Yonsei Med J. 2015; 56:1206–1212.
Article36. Nelius T, Klatte T, de Riese W, Filleur S. Impact of PSA flare-up in patients with hormone-refractory prostate cancer undergoing chemotherapy. Int Urol Nephrol. 2008; 40:97–104.
Article37. Han KS, Hong SJ. Exponential rise in prostate-specific antigen (PSA) during anti-androgen withdrawal predicts PSA flare after docetaxel chemotherapy in patients with castration-resistant prostate cancer. Yonsei Med J. 2015; 56:368–374.
Article38. Koo KC, Lee DH, Kim KH, Lee SH, Hong CH, Hong SJ, et al. Unrecognized kinetics of serum testosterone: impact on short-term androgen deprivation therapy for prostate cancer. Yonsei Med J. 2014; 55:570–575.
Article39. Thuret R, Massard C, Gross-Goupil M, Escudier B, Di Palma M, Bossi A, et al. The postchemotherapy PSA surge syndrome. Ann Oncol. 2008; 19:1308–1311.
Article40. Gillessen S, Omlin A, Attard G, de Bono JS, Efstathiou E, Fizazi K, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015; 26:1589–1604.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Systemic Therapies for Metastatic Castration-Resistant Prostate Cancer: An Updated Review
- Hormonal therapy and chemotherapy for advanced prostate cancer
- Long-Lasting Antiandrogen Withdrawal Syndrome in Castration-Resistant Prostate Cancer: Three Cases With Complete Response
- The Current Status of Metastatic Castration-Naïve Prostate Cancer Management