The Relationship between Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease Measured by Controlled Attenuation Parameter
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Liver Cirrhosis Clinical Research Center, Seoul, Korea. gihankhys@yuhs.ac
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Executive Healthcare Clinic, Severance Hospital, Yonsei Health System, Seoul, Korea.
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2374119
- DOI: http://doi.org/10.3349/ymj.2016.57.4.885
Abstract
- PURPOSE
The severity of non-alcoholic fatty liver disease (NAFLD) in type 2 diabetes mellitus (T2DM) population compared with that in normal glucose tolerance (NGT) individuals has not yet been quantitatively assessed. We investigated the prevalence and the severity of NAFLD in a T2DM population using controlled attenuation parameter (CAP).
MATERIALS AND METHODS
Subjects who underwent testing for biomarkers related to T2DM and CAP using Fibroscan® during a regular health check-up were enrolled. CAP values of 250 dB/m and 300 dB/m were selected as the cutoffs for the presence of NAFLD and for moderate to severe NAFLD, respectively. Biomarkers related to T2DM included fasting glucose/insulin, fasting C-peptide, hemoglobin A1c (HbA1c), glycoalbumin, and homeostasis model assessment of insulin resistance of insulin resistance (HOMA-IR).
RESULTS
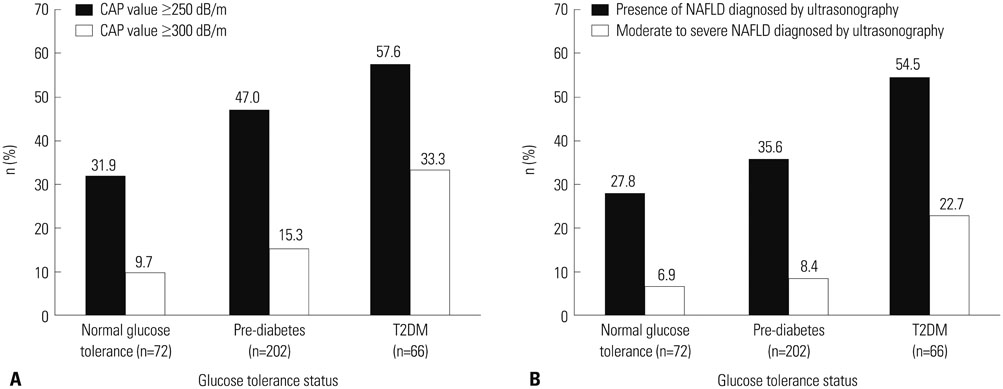
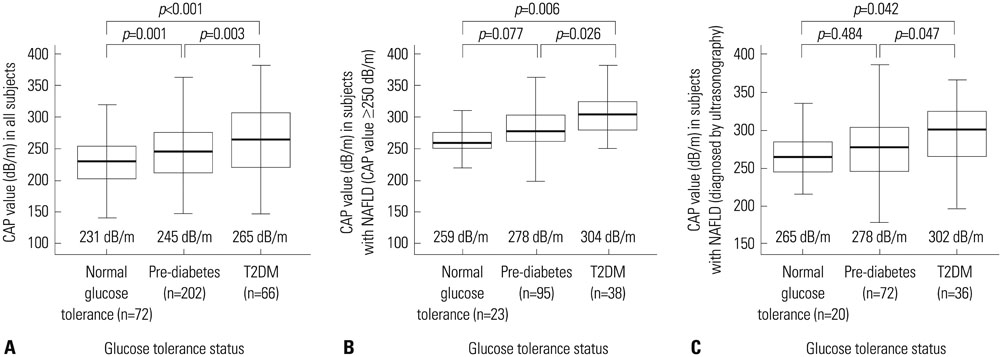
Among 340 study participants (T2DM, n=66; pre-diabetes, n=202; NGT, n=72), the proportion of subjects with NAFLD increased according to the glucose tolerance status (31.9% in NGT; 47.0% in pre-diabetes; 57.6% in T2DM). The median CAP value was significantly higher in subjects with T2DM (265 dB/m) than in those with pre-diabetes (245 dB/m) or NGT (231 dB/m) (all p<0.05). Logistic regression analysis showed that subjects with moderate to severe NAFLD had a 2.8-fold (odds ratio) higher risk of having T2DM than those without NAFLD (p=0.02; 95% confidence interval, 1.21-6.64), and positive correlations between the CAP value and HOMA-IR (Ï=0.407) or fasting C-peptide (Ï=0.402) were demonstrated.
CONCLUSION
Subjects with T2DM had a higher prevalence of severe NAFLD than those with NGT. Increased hepatic steatosis was significantly associated with the presence of T2DM, and insulin resistance induced by hepatic fat may be an important mechanistic connection.
Keyword
MeSH Terms
-
Adult
Aged
Biomarkers/metabolism
C-Peptide/metabolism
Case-Control Studies
Diabetes Mellitus, Type 2/*complications/metabolism
Female
Hemoglobin A, Glycosylated/metabolism
Humans
Insulin Resistance
Male
Middle Aged
Non-alcoholic Fatty Liver Disease/*epidemiology/metabolism/pathology
Odds Ratio
Prevalence
Biomarkers
C-Peptide
Hemoglobin A, Glycosylated
Figure
Cited by 4 articles
-
Influence of Besifovir Dipivoxil Maleate Combined with L-Carnitine on Hepatic Steatosis in Patients with Chronic Hepatitis B
Yeon Woo Jung, Moonhyun Kim, Beom Kyung Kim, Jun Yong Park, Do Young Kim, Sang Hoon Ahn, Kwang-Hyub Han, Seung Up Kim
J Korean Med Sci. 2020;35(17):e104. doi: 10.3346/jkms.2020.35.e104.Radiologic Evaluation of Non-Alcoholic Fatty Liver Disease in Diabetic Patient
Kwang Joon Kim, Seung Up Kim, Yong Eun Chung, Chang Oh Kim
J Korean Diabetes. 2017;18(2):88-101. doi: 10.4093/jkd.2017.18.2.88.Effect of Dapagliflozin on Alanine Aminotransferase Improvement in Type 2 Diabetes Mellitus with Non-alcoholic Fatty Liver Disease
Dug-Hyun Choi, Chan-Hee Jung, Ji-Oh Mok, Chul-Hee Kim, Sung-Koo Kang, Bo-Yeon Kim
Endocrinol Metab. 2018;33(3):387-394. doi: 10.3803/EnM.2018.33.3.387.Pathologic Impact of Insulin Resistance and Sensitivity on the Severity of Liver Histopathology in Pediatric Non-Alcoholic Steatohepatitis
Byung Han Park, Jung Min Yoon, Ja Hye Kim, Jin-Hwa Moon, Young Ho Lee, Se Min Jang, Yong Joo Kim
Yonsei Med J. 2017;58(4):756-762. doi: 10.3349/ymj.2017.58.4.756.
Reference
-
1. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001; 50:1844–1850.2. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
Article3. Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003; 38:954–961.
Article4. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004; 40:1387–1395.
Article5. Kim HC, Choi KS, Jang YH, Shin HW, Kim DJ. Normal serum aminotransferase levels and the metabolic syndrome: Korean National Health and Nutrition Examination Surveys. Yonsei Med J. 2006; 47:542–550.
Article6. Moore JB. Non-alcoholic fatty liver disease: the hepatic consequence of obesity and the metabolic syndrome. Proc Nutr Soc. 2010; 69:211–220.
Article7. Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008; 28:27–38.8. Sanyal AJ. American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002; 123:1705–1725.
Article9. Lee J, Hong SW, Rhee EJ, Lee WY. GLP-1 Receptor agonist and nonalcoholic fatty liver disease. Diabetes Metab J. 2012; 36:262–267.
Article10. Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004; 164:2169–2175.
Article11. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013; 10:330–344.
Article12. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013; 57:1378–1383.
Article13. Bae JC, Rhee EJ, Lee WY, Park SE, Park CY, Oh KW, et al. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: a 4-year retrospective longitudinal study. Diabetes Care. 2011; 34:727–729.
Article14. Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007; 30:2940–2944.
Article15. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002; 123:745–750.
Article16. Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010; 36:1825–1835.
Article17. Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012; 32:902–910.
Article18. Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012; 19:244–253.
Article19. Kumar M, Rastogi A, Singh T, Behari C, Gupta E, Garg H, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance. J Gastroenterol Hepatol. 2013; 28:1194–1201.
Article20. Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim do Y, et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014; 34:102–109.
Article21. American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012; 35:Suppl 1. S11–S63.22. Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed). 1986; 292:13–15.
Article23. Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008; 48:835–847.
Article24. Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005; 22:1141–1145.
Article25. Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007; 17:863–869.
Article26. de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014; 60:1026–1031.
Article27. Barma P, Bhattacharya S, Bhattacharya A, Kundu R, Dasgupta S, Biswas A, et al. Lipid induced overexpression of NF-kappaB in skeletal muscle cells is linked to insulin resistance. Biochim Biophys Acta. 2009; 1792:190–200.
Article28. Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002; 87:3023–3028.
Article29. Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005; 48:634–642.
Article30. Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward-Sadler H, et al. Hepatic changes in the failing Fontan circulation. Heart. 2007; 93:579–584.
Article31. Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, et al. Prevalence of Diabetes and Prediabetes according to Fasting Plasma Glucose and HbA1c. Diabetes Metab J. 2013; 37:349–357.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between Controlled Attenuation Parameter and Hepatic Steatosis as Assessed by Ultrasound in Alcoholic or Nonalcoholic Fatty Liver Disease
- A Case of Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease
- The Effects of Hypoglycemic Agents on Non-alcoholic Fatty Liver Disease: Focused on Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists
- Tamoxifen-Induced Non-alcoholic Steatohepatitis Cirrhosis
- Nonalcoholic Fatty Liver Disease Is a Stepping Stone in the Path toward Diabetes Mellitus