Yonsei Med J.
2016 Jul;57(4):817-823. 10.3349/ymj.2016.57.4.817.
Hearing Restoration in Neurofibromatosis Type II Patients
- Affiliations
-
- 1Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea. ismoonmd@yuhs.ac
- 2Department of Neurosurgery, Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea. changws0716@yuhs.ac
- KMID: 2374110
- DOI: http://doi.org/10.3349/ymj.2016.57.4.817
Abstract
- Patients with neurofibromatosis type II will eventually succumb to bilateral deafness. For patients with hearing loss, modern medical science technology can provide efficient hearing restoration through a number of various methods. In this article, several hearing restoration methods for patients with neurofibromatosis type II are introduced.
Keyword
MeSH Terms
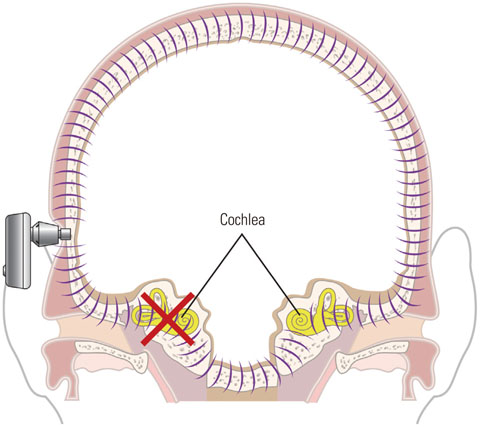
Figure
Reference
-
1. Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005; 26:93–97.
Article2. Evans DG, Huson SM, Donnai D, Neary W, Blair V, Newton V, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992; 84:603–618.3. Asthagiri AR, Vasquez RA, Butman JA, Wu T, Morgan K, Brewer CC, et al. Mechanisms of hearing loss in neurofibromatosis type 2. PLoS One. 2012; 7:e46132.
Article4. Masuda A, Fisher LM, Oppenheimer ML, Iqbal Z, Slattery WH. Natural History Consortium. Hearing changes after diagnosis in neurofibromatosis type 2. Otol Neurotol. 2004; 25:150–154.
Article5. Graamans K, Van Dijk JE, Janssen LW. Hearing deterioration in patients with a non-growing vestibular schwannoma. Acta Otolaryngol. 2003; 123:51–54.
Article6. Roosli C, Linthicum FH Jr, Cureoglu S, Merchant SN. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: an underappreciated entity. Otol Neurotol. 2012; 33:473–480.
Article7. Silverstein H. Labyrinthine tap as a diagnostic test for acoustic neurinoma. Otolaryngol Clin North Am. 1973; 6:229–244.
Article8. Gates GA, Cooper JC Jr, Kannel WB, Miller NJ. Hearing in the elderly: the Framingham cohort, 1983-1985. Part I. Basic audiometric test results. Ear Hear. 1990; 11:247–256.9. Flint PW, Haughey BH, Lund V, Niparko JK, Robbins KT, Thomas JR, et al. Cummings otolaryngology. Philadelphia, PA: Elsevier;2015.10. Lotterman SH, Kasten RN. Examination of the CROS type hearing aid. J Speech Hear Res. 1971; 14:416–420.
Article11. Lin LM, Bowditch S, Anderson MJ, May B, Cox KM, Niparko JK. Amplification in the rehabilitation of unilateral deafness: speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otol Neurotol. 2006; 27:172–182.
Article12. Laske RD, Röösli C, Pfiffner F, Veraguth D, Huber AM. Functional results and subjective benefit of a transcutaneous bone conduction device in patients with single-sided deafness. Otol Neurotol. 2015; 36:1151–1156.
Article13. Snapp H, Angeli S, Telischi FF, Fabry D. Postoperative validation of bone-anchored implants in the single-sided deafness population. Otol Neurotol. 2012; 33:291–296.
Article14. Berger KW. Early bone conduction hearing aid devices. Arch Otolaryngol. 1976; 102:315–318.
Article15. Kompis M, Kurz A, Pfiffner F, Senn P, Arnold A, Caversaccio M. Is complex signal processing for bone conduction hearing aids useful? Cochlear Implants Int. 2014; 15:Suppl 1. S47–S50.
Article16. Dun CA, Faber HT, de Wolf MJ, Mylanus EA, Cremers CW, Hol MK. Assessment of more than 1,000 implanted percutaneous bone conduction devices: skin reactions and implant survival. Otol Neurotol. 2012; 33:192–198.
Article17. Lassaletta L, Sanchez-Cuadrado I, Muñoz E, Gavilan J. Retrosigmoid implantation of an active bone conduction stimulator in a patient with chronic otitis media. Auris Nasus Larynx. 2014; 41:84–87.
Article18. Hol MK, Cremers CW, Coppens-Schellekens W, Snik AF. The BAHA Softband. A new treatment for young children with bilateral congenital aural atresia. Int J Pediatr Otorhinolaryngol. 2005; 69:973–980.19. Doshi J, McDermott AL. Bone anchored hearing aids in children. Expert Rev Med Devices. 2015; 12:73–82.
Article20. Siegert R. Partially implantable bone conduction hearing aids without a percutaneous abutment (Otomag): technique and preliminary clinical results. Adv Otorhinolaryngol. 2011; 71:41–46.
Article21. Heywood RL, Patel PM, Jonathan DA. Comparison of hearing thresholds obtained with Baha preoperative assessment tools and those obtained with the osseointegrated implant. Ear Nose Throat J. 2011; 90:E21–E27.
Article22. Kurz A, Flynn M, Caversaccio M, Kompis M. Speech understanding with a new implant technology: a comparative study with a new nonskin penetrating Baha system. Biomed Res Int. 2014; 2014:416205.
Article23. Reinfeldt S, Håkansson B, Taghavi H, Eeg-Olofsson M. New developments in bone-conduction hearing implants: a review. Med Devices (Auckl). 2015; 8:79–93.
Article24. Jansson KJ, Håkansson B, Reinfeldt S, Rigato C, Eeg-Olofsson M. Magnetic resonance imaging investigation of the bone conduction implant - a pilot study at 1.5 Tesla. Med Devices (Auckl). 2015; 8:413–423.25. Rha MS, Jeong SW, Seo YW, Moon IS. Hearing rehabilitation with Sophono® in patients with unilateral hearing loss after meningioma removal. Korean J Otorhinolaryngol-Head Neck Surg. 2015; 58:514–519.
Article26. Steinmetz C, Mader I, Arndt S, Aschendorff A, Laszig R, Hassepass F. MRI artefacts after Bonebridge implantation. Eur Arch Otorhinolaryngol. 2014; 271:2079–2082.
Article27. Toh EH, Luxford WM. Cochlear and brainstem implantation. 2002. Neurosurg Clin N Am. 2008; 19:317–329. vii.28. Carlson ML, Breen JT, Driscoll CL, Link MJ, Neff BA, Gifford RH, et al. Cochlear implantation in patients with neurofibromatosis type 2: variables affecting auditory performance. Otol Neurotol. 2012; 33:853–862.29. Hoffman RA, Kohan D, Cohen NL. Cochlear implants in the management of bilateral acoustic neuromas. Am J Otol. 1992; 13:525–528.30. Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2013; 119:Suppl. 745–759.
Article31. Lustig LR, Yeagle J, Driscoll CL, Blevins N, Francis H, Niparko JK. Cochlear implantation in patients with neurofibromatosis type 2 and bilateral vestibular schwannoma. Otol Neurotol. 2006; 27:512–518.
Article32. Evans DG, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet. 2006; 43:289–294.
Article33. Majdani O, Leinung M, Rau T, Akbarian A, Zimmerling M, Lenarz M, et al. Demagnetization of cochlear implants and temperature changes in 3.0T MRI environment. Otolaryngol Head Neck Surg. 2008; 139:833–839.
Article34. Crane BT, Gottschalk B, Kraut M, Aygun N, Niparko JK. Magnetic resonance imaging at 1.5 T after cochlear implantation. Otol Neurotol. 2010; 31:1215–1220.
Article35. Kim BG, Kim JW, Park JJ, Kim SH, Kim HN, Choi JY. Adverse events and discomfort during magnetic resonance imaging in cochlear implant recipients. JAMA Otolaryngol Head Neck Surg. 2015; 141:45–52.
Article36. Kim JW, Moon IS. Simultaneous translabyrinthine tumor removal and cochlear implantation in vestibular schwannoma patients. Yonsei Med J. 2016; In press.
Article37. Linthicum FH Jr, Brackmann DE. Bilateral acoustic tumors. A diagnostic and surgical challenge. Arch Otolaryngol. 1980; 106:729–733.
Article38. Lambert PR, Ruth RA, Thomas JF. Promontory electrical stimulation in postoperative acoustic tumor patients. Laryngoscope. 1992; 102:814–819.
Article39. Chamoun R, MacDonald J, Shelton C, Couldwell WT. Surgical approaches for resection of vestibular schwannomas: translabyrinthine, retrosigmoid, and middle fossa approaches. Neurosurg Focus. 2012; 33:E9.
Article40. Cole T, Veeravagu A, Zhang M, Azad T, Swinney C, Li GH, et al. Retrosigmoid versus translabyrinthine approach for acoustic neuroma resection: an assessment of complications and payments in a longitudinal administrative database. Cureus. 2015; 7:e369.
Article41. Neff BA, Wiet RM, Lasak JM, Cohen NL, Pillsbury HC, Ramsden RT, et al. Cochlear implantation in the neurofibromatosis type 2 patient: long-term follow-up. Laryngoscope. 2007; 117:1069–1072.
Article42. Arístegui M, Denia A. Simultaneous cochlear implantation and translabyrinthine removal of vestibular schwannoma in an only hearing ear: report of two cases (neurofibromatosis type 2 and unilateral vestibular schwannoma). Otol Neurotol. 2005; 26:205–210.
Article43. Vincenti V, Pasanisi E, Guida M, Di Trapani G, Sanna M. Hearing rehabilitation in neurofibromatosis type 2 patients: cochlear versus auditory brainstem implantation. Audiol Neurootol. 2008; 13:273–280.
Article44. Tran Ba Huy P, Kania R, Frachet B, Poncet C, Legac MS. Auditory rehabilitation with cochlear implantation in patients with neurofibromatosis type 2. Acta Otolaryngol. 2009; 129:971–975.
Article45. Celis-Aguilar E, Lassaletta L, Gavilán J. Cochlear implantation in patients with neurofibromatosis type 2 and patients with vestibular schwannoma in the only hearing ear. Int J Otolaryngol. 2012; 2012:157497.
Article46. Choi JY, Song MH, Jeon JH, Lee WS, Chang JW. Early surgical results of auditory brainstem implantation in nontumor patients. Laryngoscope. 2011; 121:2610–2618.
Article47. Bernardeschi D, Peyre M, Collin M, Smail M, Sterkers O, Kalamarides M. Internal auditory canal decompression for hearing maintenance in neurofibromatosis type 2 patients. Neurosurgery. 2015; 11. 16. DOI: 10.1227/neu.0000000000001125. [Epub].
Article48. Monsell EM, McElveen JT Jr, Hitselberger WE, House WF. Surgical approaches to the human cochlear nuclear complex. Am J Otol. 1987; 8:450–455.49. Waring MD. Intraoperative electrophysiologic monitoring to assist placement of auditory brain stem implant. Ann Otol Rhinol Laryngol Suppl. 1995; 166:33–36.50. Lenarz M, Matthies C, Lesinski-Schiedat A, Frohne C, Rost U, Illg A, et al. Auditory brainstem implant part II: subjective assessment of functional outcome. Otol Neurotol. 2002; 23:694–697.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Neurofibromatosis Type II
- An Experience of Early Surgical Intervention for Neurofibromatosis Type II
- Decompression of Internal Auditory Canal via Middle Fossa Approach in Neurofibromatosis Type II with Only Hearing Ear
- A Case of Cochlear Implantation in the Patient With Neurofibromatosis Type II Considering Magnetic Resonance Imaging
- Multiple Cutaneous Plexiform Schwannomas as the First Symptom of Neurofibromatosis Type 2