Yonsei Med J.
2016 Mar;57(2):283-290. 10.3349/ymj.2016.57.2.283.
A Simple, Reproducible, Inexpensive, Yet Old-Fashioned Method for Determining Phagocytic and Bactericidal Activities of Macrophages
- Affiliations
-
- 1Laboratory of Immunology, Department of Laboratory Sciences, Gunma University Graduate School of Health Sciences, Maebashi, Japan. masashi.emoto@gmail.com
- KMID: 2374030
- DOI: http://doi.org/10.3349/ymj.2016.57.2.283
Abstract
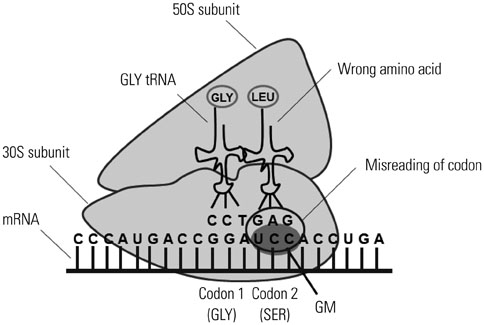
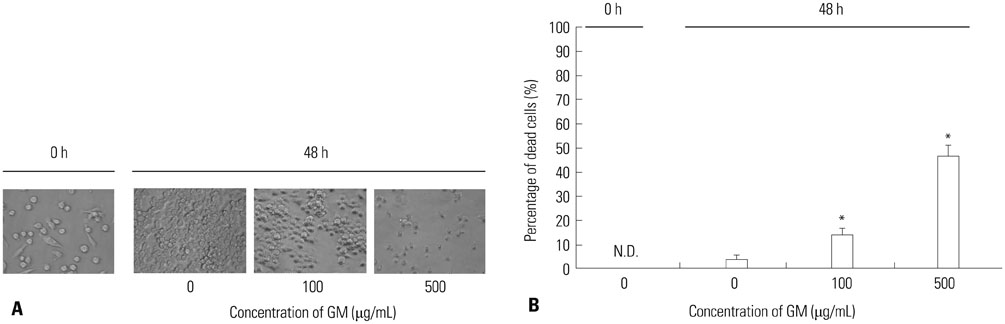
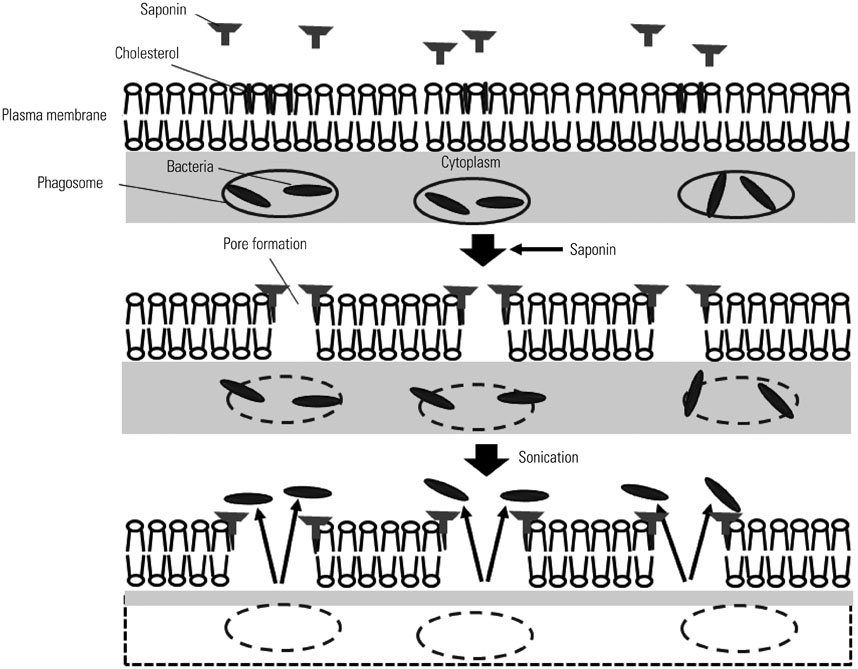
- Macrophages (Mphi) play a pivotal role in the protection system by recognizing and eliminating invading pathogenic bacteria. Phagocytosis and the killing of invading bacteria are major effector functions of Mphi. Although the phagocytic and bactericidal activities of Mphi have been analyzed via several methods using a light microscope, a fluorescence microscope, or a fluorescence-activated cell sorter, expensive materials and equipment are usually required, and the methods are rather complicated. Moreover, it is impossible to determine both the phagocytic and bactericidal activities of Mphi simultaneously using these methods. In this review, we describe a simple, reproducible, inexpensive, yet old-fashioned method (antibiotic protection assay) for determining the phagocytic and bactericidal activities of Mphi.
Keyword
MeSH Terms
Figure
Reference
-
1. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009; 78:857–902.
Article2. Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012; 12:492–502.
Article3. Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, et al. The phagosome proteome: insight into phagosome functions. J Cell Biol. 2001; 152:165–180.4. Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004; 2:820–832.
Article5. Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol. 2009; 7:333–340.
Article6. Buchmeier NA, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989; 57:1–7.
Article7. Skerrett SJ, Martin TR. Recombinant murine interferon-gamma reversibly activates rat alveolar macrophages to kill Legionella pneumophila. J Infect Dis. 1992; 166:1354–1361.
Article8. Inoue S, Itagaki S, Amano F. Intracellular killing of Listeria monocytogenes in the J774.1 macrophage-like cell line and the lipopolysaccharide (LPS)-resistant mutant LPS1916 cell line defective in the generation of reactive oxygen intermediates after LPS treatment. Infect Immun. 1995; 63:1876–1886.
Article9. Stevanin TM, Moir JW, Read RC. Nitric oxide detoxification systems enhance survival of Neisseria meningitidis in human macrophages and in nasopharyngeal mucosa. Infect Immun. 2005; 73:3322–3329.
Article10. Emoto M, Yoshida T, Fukuda T, Kawamura I, Mitsuyama M, Kita E, et al. Alpha-galactosylceramide promotes killing of Listeria monocytogenes within the macrophage phagosome through invariant NKT-cell activation. Infect Immun. 2010; 78:2667–2676.
Article11. Mandell GL. Interaction of intraleukocytic bacteria and antibiotics. J Clin Invest. 1973; 52:1673–1679.
Article12. Lobo MC, Mandell GL. The effect of antibiotics on Escherichia coli ingested by macrophages. Proc Soc Exp Biol Med. 1973; 142:1048–1050.13. Vaudaux P, Waldvogel FA. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1979; 16:743–749.
Article14. Bryskier A. Antimicrobial agents. 1st ed. Washington, DC: ASM Press;2005.15. Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev. 2003; 16:430–450.
Article16. Klein JO, Eickhoff TC, Finland M. Gentamicin: activity in vitro and observations in 26 patients. Am J Med Sci. 1964; 248:528–544.17. Waitz JA, Weinstein MJ. Recent microbiological studies with gentamicin. J Infect Dis. 1969; 119:355–360.
Article18. Espaze EP, Reynaud AE. Antibiotic susceptibilities of Listeria: in vitro studies. Infection. 1988; 16:Suppl 2. S160–S164.19. Ho YI, Chan CY, Cheng AF. In-vitro activities of aminoglycoside-aminocyclitols against mycobacteria. J Antimicrob Chemother. 1997; 40:27–32.
Article20. Wilson G, Easow JM, Mukhopadhyay C, Shivananda PG. Isolation & antimicrobial susceptibility of Shigella from patients with acute gastroenteritis in Western Nepal. Indian J Med Res. 2006; 123:145–150.21. Schmid S, Knoblauch K. Digestive Organs. In : Bühlmann AA, Froesch ER, editors. Pathophysiology. 1st ed. New York: Springer-Verlag;1979. p. 261–315.22. Tseng JT, Bryan LE, Van den Elzen HM. Mechanisms and spectrum of streptomycin resistance in a natural population of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1972; 2:136–141.
Article23. Ximenes J, Bassoi ON, de Menezes JP, Fry W. Activity of amikacin, gentamicin and kanamycin against Pseudomonas aeruginosa. J Int Med Res. 1976; 4:165–175.
Article24. de Melo MA, Pechère JC. Effect of mucin on Campylobacter jejuni association and invasion on HEp-2 cells. Microb Pathog. 1988; 5:71–76.
Article25. Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988; 167:1459–1471.
Article26. Shaw JH, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988; 56:1625–1632.
Article27. Drevets DA, Canono BP, Leenen PJ, Campbell PA. Gentamicin kills intracellular Listeria monocytogenes. Infect Immun. 1994; 62:2222–2228.
Article28. Ohya S, Xiong H, Tanabe Y, Arakawa M, Mitsuyama M. Killing mechanism of Listeria monocytogenes in activated macrophages as determined by an improved assay system. J Med Microbiol. 1998; 47:211–215.
Article29. Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002; 88:587–605.
Article30. Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971; 134(3 Pt 1):713–740.
Article31. Herbst S, Schaible UE, Schneider BE. Interferon gamma activated macrophages kill mycobacteria by nitric oxide induced apoptosis. PLoS One. 2011; 6:e19105.
Article32. Drevets DA, Campbell PA. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect Immun. 1991; 59:2645–2652.
Article33. Drevets DA, Canono BP, Campbell PA. Listericidal and nonlistericidal mouse macrophages differ in complement receptor type 3-mediated phagocytosis of L. monocytogenes and in preventing escape of the bacteria into the cytoplasm. J Leukoc Biol. 1992; 52:70–79.
Article34. Utermöhlen O, Karow U, Löhler J, Krönke M. Severe impairment in early host defense against Listeria monocytogenes in mice deficient in acid sphingomyelinase. J Immunol. 2003; 170:2621–2628.
Article35. Sharma L, Wu W, Dholakiya SL, Gorasiya S, Wu J, Sitapara R, et al. Assessment of phagocytic activity of cultured macrophages using fluorescence microscopy and flow cytometry. Methods Mol Biol. 2014; 1172:137–145.
Article36. Kaneko M, Kanayama Y, Emoto Y, Emoto M. Several methods for determination of phagocytic and killing activities of macrophages against Listeria monocytogenes. In : Vicario T, editor. Listeria monocytogenes: incidence, growth behavior and control. 1st ed. New York: Nova Science Publishers;2015. p. 15363–163.37. Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods. 1985; 82:131–140.
Article38. Mancuso P, Peters-Golden M, Goel D, Goldberg J, Brock TG, Greenwald-Yarnell M, et al. Disruption of leptin receptor-STAT3 signaling enhances leukotriene production and pulmonary host defense against pneumococcal pneumonia. J Immunol. 2011; 186:1081–1090.
Article39. Domingo-Gonzalez R, Katz S, Serezani CH, Moore TA, Levine AM, Moore BB. Prostaglandin E2-induced changes in alveolar macrophage scavenger receptor profiles differentially alter phagocytosis of Pseudomonas aeruginosa and Staphylococcus aureus post-bone marrow transplant. J Immunol. 2013; 190:5809–5817.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endogenous interferon ?alpha)/?beta) produced by lipid A-stimulated macrophages enganced phagocytic activity of mouse macrophages
- Sulfatase 1 and sulfatase 2 as novel regulators of macrophage antigen presentation and phagocytosis
- Macrophages in the Corpus Luteum of the Rat : Immunohistochemical and Transmission Electron Microscopic Study
- Influence of Mycoplasma Pneumoniae Infection on the Growth, Phagocytie Activities and Induction of Nitric Oxide Production of the Microglial Cells of Mice
- Attempts to Establish Host Cells for Mycobactrium leprae in vitro by Hibridizing Mouse Macrophages and HeLa Cells