Yeungnam Univ J Med.
2021 Oct;38(4):326-336. 10.12701/yujm.2021.01025.
Sulfatase 1 and sulfatase 2 as novel regulators of macrophage antigen presentation and phagocytosis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, CHA University, CHA Gumi Medical Center, Gumi, Korea
- 2Department of Microbiology, Yeungnam University College of Medicine, Daegu, Korea
- 3Division of Rheumatology, Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
- KMID: 2521712
- DOI: http://doi.org/10.12701/yujm.2021.01025
Abstract
- Background
Sulfation of heparan sulfate proteoglycans (HSPGs) is critical for the binding and signaling of ligands that mediate inflammation. Extracellular 6-O-endosulfatases regulate posttranslational sulfation levels and patterns of HSPGs. In this study, extracellular 6-O-endosulfatases, sulfatase (Sulf)-1 and Sulf-2, were evaluated for their expression and function in inflammatory cells and tissues.
Methods
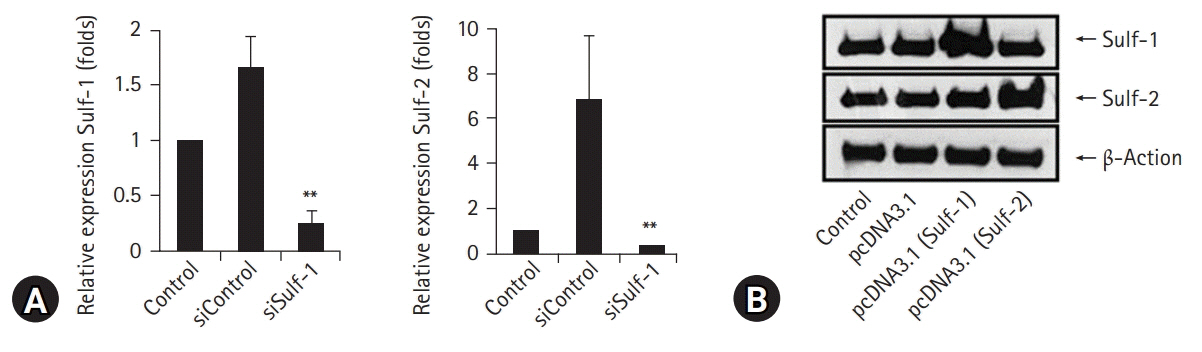
Harvested human peripheral blood mononuclear cells were treated with phytohemagglutinin and lipopolysaccharide, and murine peritoneal macrophages were stimulated with interleukin (IL)-1β for the evaluation of Sulf-1 and Sulf-2 expression. Sulf expression in inflammatory cells was examined in the human rheumatoid arthritis (RA) synovium by immunofluorescence staining. The antigen presentation and phagocytic activities of macrophages were compared according to the expression state of Sulfs. Sulfs-knockdown macrophages and Sulfs-overexpressing macrophages were generated using small interfering RNAs and pcDNA3.1 plasmids for Sulf-1 and Sulf-2, respectively.
Results
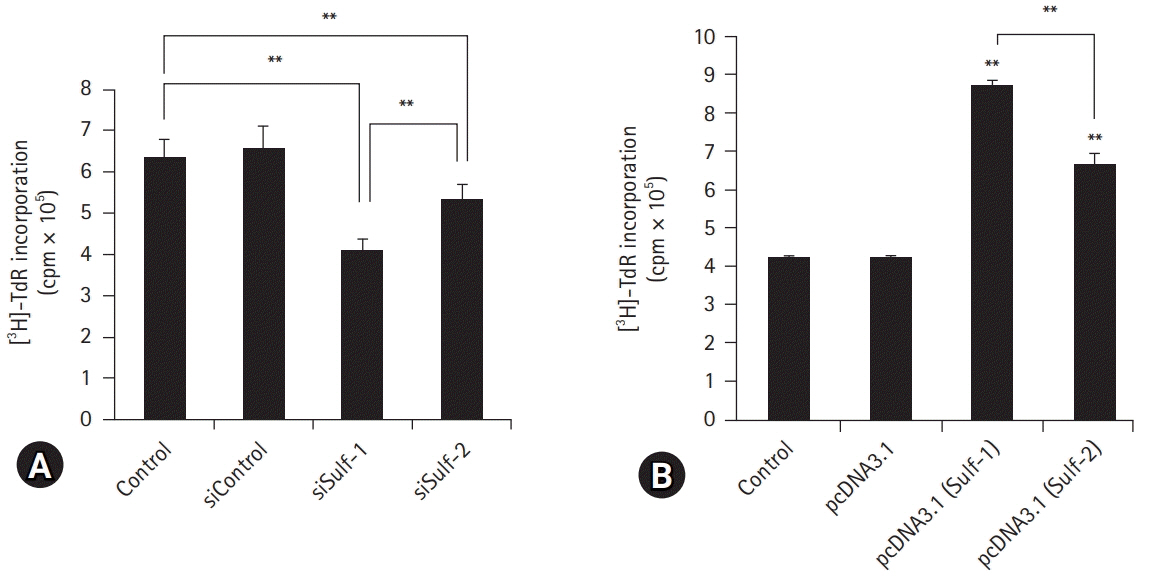
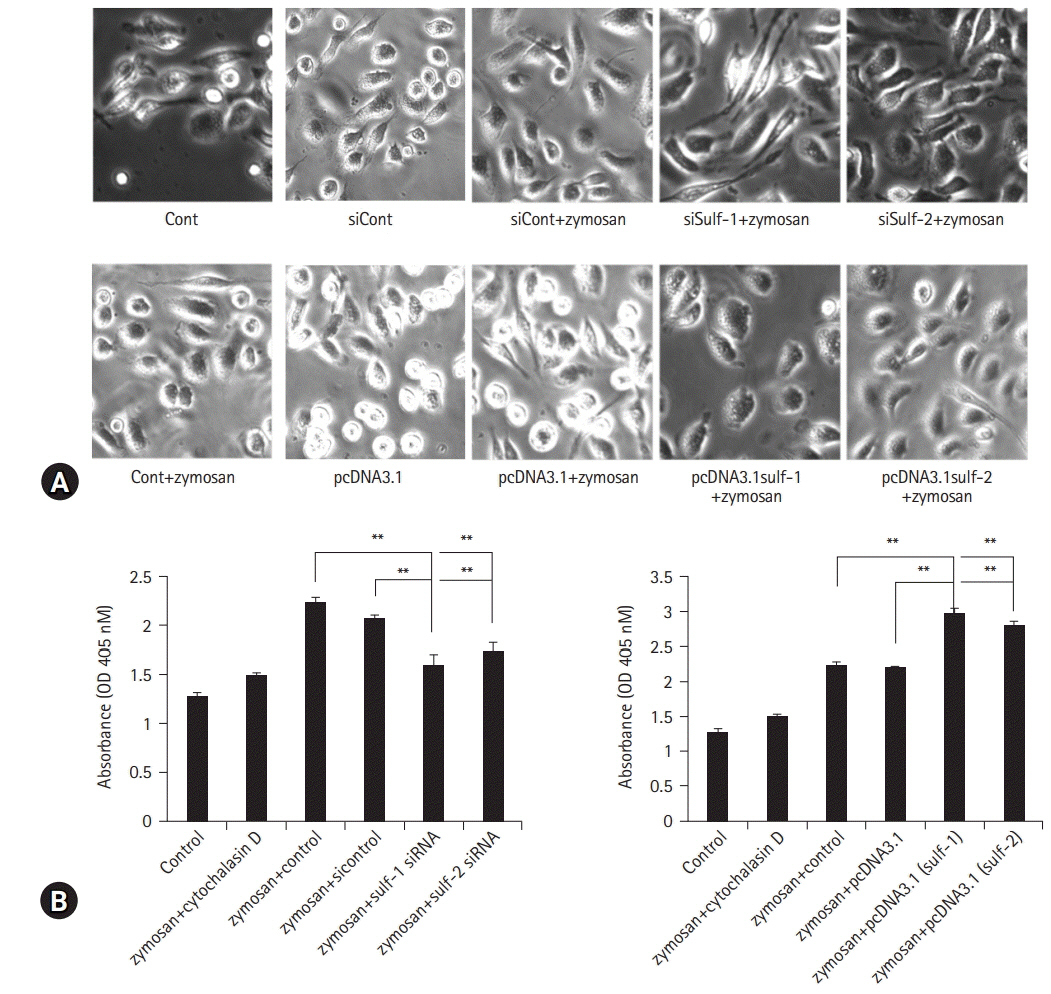
Lymphocytes and monocytes showed weak Sulf expression, which remained unaffected by IL-1β. However, peritoneal macrophages showed increased expression of Sulfs upon stimulation with IL-1β. In human RA synovium, two-colored double immunofluorescent staining of Sulfs and CD68 revealed active upregulation of Sulfs in macrophages of inflamed tissues, but not in lymphocytes of lymphoid follicles. Macrophages are professional antigen-presenting cells. The antigen presentation and phagocytic activities of macrophages were dependent on the level of Sulf expression, suppressed in Sulfs-knockdown macrophages, and enhanced in Sulfs-overexpressing macrophages.
Conclusion
The results demonstrate that upregulation of Sulfs in macrophages occurs in response to inflammation, and Sulfs actively regulate the antigen presentation and phagocytic activities of macrophages as novel immune regulators.
Keyword
Figure
Reference
-
References
1. Dreyfuss JL, Regatieri CV, Jarrouge TR, Cavalheiro RP, Sampaio LO, Nader HB. Heparan sulfate proteoglycans: structure, protein interactions and cell signaling. An Acad Bras Cienc. 2009; 81:409–29.
Article2. Clasper S, Vekemans S, Fiore M, Plebanski M, Wordsworth P, David G, et al. Inducible expression of the cell surface heparan sulfate proteoglycan syndecan-2 (fibroglycan) on human activated macrophages can regulate fibroblast growth factor action. J Biol Chem. 1999; 274:24113–23.
Article3. Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006; 6:633–43.
Article4. Vanpouille C, Deligny A, Delehedde M, Denys A, Melchior A, Liénard X, et al. The heparin/heparan sulfate sequence that interacts with cyclophilin B contains a 3-O-sulfated N-unsubstituted glucosamine residue. J Biol Chem. 2007; 282:24416–29.
Article5. Matsuo I, Kimura-Yoshida C. Extracellular modulation of fibroblast growth factor signaling through heparan sulfate proteoglycans in mammalian development. Curr Opin Genet Dev. 2013; 23:399–407.
Article6. Witt DP, Lander AD. Differential binding of chemokines to glycosaminoglycan subpopulations. Curr Biol. 1994; 4:394–400.
Article7. Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005; 6:902–10.
Article8. Rops AL, van den Hoven MJ, Baselmans MM, Lensen JF, Wijnhoven TJ, van den Heuvel LP, et al. Heparan sulfate domains on cultured activated glomerular endothelial cells mediate leukocyte trafficking. Kidney Int. 2008; 73:52–62.
Article9. Kawashima H. Roles of sulfated glycans in lymphocyte homing. Biol Pharm Bull. 2006; 29:2343–9.
Article10. van der Voort R, Keehnen RM, Beuling EA, Spaargaren M, Pals ST. Regulation of cytokine signaling by B cell antigen receptor and CD40-controlled expression of heparan sulfate proteoglycans. J Exp Med. 2000; 192:1115–24.
Article11. Floris S, van den Born J, van der Pol SM, Dijkstra CD, De Vries HE. Heparan sulfate proteoglycans modulate monocyte migration across cerebral endothelium. J Neuropathol Exp Neurol. 2003; 62:780–90.
Article12. Celie JW, Rutjes NW, Keuning ED, Soininen R, Heljasvaara R, Pihlajaniemi T, et al. Subendothelial heparan sulfate proteoglycans become major L-selectin and monocyte chemoattractant protein-1 ligands upon renal ischemia/reperfusion. Am J Pathol. 2007; 170:1865–78.
Article13. Diez-Roux G, Ballabio A. Sulfatases and human disease. Annu Rev Genomics Hum Genet. 2005; 6:355–79.
Article14. Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP Jr. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001; 293:1663–6.
Article15. Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002; 277:49175–85.
Article16. Ohto T, Uchida H, Yamazaki H, Keino-Masu K, Matsui A, Masu M. Identification of a novel nonlysosomal sulphatase expressed in the floor plate, choroid plexus and cartilage. Genes Cells. 2002; 7:173–85.
Article17. Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP Jr. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol. 2003; 162:341–51.
Article18. Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999; 68:729–77.
Article19. Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001; 108:169–73.
Article20. Jackson DG. Human leucocyte heparan sulphate proteoglycans and their roles in inflammation. Biochem Soc Trans. 1997; 25:220–4.
Article21. Camp RL, Scheynius A, Johansson C, Puré E. CD44 is necessary for optimal contact allergic responses but is not required for normal leukocyte extravasation. J Exp Med. 1993; 178:497–507.
Article22. Mikecz K, Brennan FR, Kim JH, Glant TT. Anti-CD44 treatment abrogates tissue oedema and leukocyte infiltration in murine arthritis. Nat Med. 1995; 1:558–63.
Article23. Kim JH, Chan C, Elwell C, Singer MS, Dierks T, Lemjabbar-Alaoui H, et al. Endosulfatases SULF1 and SULF2 limit Chlamydia muridarum infection. Cell Microbiol. 2013; 15:1560–71.
Article24. Habuchi H, Habuchi O, Kimata K. Sulfation pattern in glycosaminoglycan: does it have a code? Glycoconj J. 2004; 21:47–52.
Article25. Nawroth R, van Zante A, Cervantes S, McManus M, Hebrok M, Rosen SD. Extracellular sulfatases, elements of the Wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PLoS One. 2007; 2:e392.
Article26. Lemjabbar-Alaoui H, van Zante A, Singer MS, Xue Q, Wang YQ, Tsay D, et al. Sulf-2, a heparan sulfate endosulfatase, promotes human lung carcinogenesis. Oncogene. 2010; 29:635–46.
Article27. Bret C, Moreaux J, Schved JF, Hose D, Klein B. SULFs in human neoplasia: implication as progression and prognosis factors. J Transl Med. 2011; 9:72.
Article28. Yang JD, Sun Z, Hu C, Lai J, Dove R, Nakamura I, et al. Sulfatase 1 and sulfatase 2 in hepatocellular carcinoma: associated signaling pathways, tumor phenotypes, and survival. Genes Chromosomes Cancer. 2011; 50:122–35.
Article29. Nagamine S, Koike S, Keino-Masu K, Masu M. Expression of a heparan sulfate remodeling enzyme, heparan sulfate 6-O-endosulfatase sulfatase FP2, in the rat nervous system. Brain Res Dev Brain Res. 2005; 159:135–43.
Article30. Buono M, Visigalli I, Bergamasco R, Biffi A, Cosma MP. Sulfatase modifying factor 1-mediated fibroblast growth factor signaling primes hematopoietic multilineage development. J Exp Med. 2010; 207:1647–60.
Article31. Langsdorf A, Schumacher V, Shi X, Tran T, Zaia J, Jain S, et al. Expression regulation and function of heparan sulfate 6-O-endosulfatases in the spermatogonial stem cell niche. Glycobiology. 2011; 21:152–61.
Article32. Otsuki S, Taniguchi N, Grogan SP, D'Lima D, Kinoshita M, Lotz M. Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res Ther. 2008; 10:R61.
Article33. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011; 11:723–37.
Article34. Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015; 15:203–16.
Article35. Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002; 20:621–67.
Article36. Léonetti M, Gadzinski A, Moine G. Cell surface heparan sulfate proteoglycans influence MHC class II-restricted antigen presentation. J Immunol. 2010; 185:3847–56.
Article37. Dehio C, Freissler E, Lanz C, Gómez-Duarte OG, David G, Meyer TF. Ligation of cell surface heparan sulfate proteoglycans by antibody-coated beads stimulates phagocytic uptake into epithelial cells: a model for cellular invasion by Neisseria gonorrhoeae. Exp Cell Res. 1998; 242:528–39.38. Reis CR, Chen PH, Srinivasan S, Aguet F, Mettlen M, Schmid SL. Crosstalk between Akt/GSK3β signaling and dynamin-1 regulates clathrin-mediated endocytosis. EMBO J. 2015; 34:2132–46.
Article39. van Deurs B, Röpke C, Thorball N. Kinetics of pinocytosis studied by flow cytometry. Eur J Cell Biol. 1984; 34:96–102.40. Ishimoto H, Yanagihara K, Araki N, Mukae H, Sakamoto N, Izumikawa K, et al. Single-cell observation of phagocytosis by human blood dendritic cells. Jpn J Infect Dis. 2008; 61:294–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of x-linked ichthyosis and detection of its carriers with southern blot hybidization
- Induction of Integrin Signaling by Steroid Sulfatase in Human Cervical Cancer Cells
- Two Cases of Pre-descemet Corneal Dystrophy Associated with X-linked Ichthyosis: A Case Report by Genetic Analysis
- Changes in Glycogen and Glycosaminoglycan Levels in Hepatocytes of Iduronate-2-Sulfatase Knockout Mice before and after Recombinant Iduronate-2-Sulfatase Supplementation
- Comparison of Macrophage Activation and Tumor - cytotoxicity in Mouse and hamster Peritoneal Macrophages by Cold Stress