J Korean Med Sci.
2016 Jul;31(7):1127-1135. 10.3346/jkms.2016.31.7.1127.
Effect of Hydroxychloroquine Treatment on Dry Eyes in Subjects with Primary Sjögren's Syndrome: a Double-Blind Randomized Control Study
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Hospital, Seoul, Korea. kmk9@snu.ac.kr
- 2Laboraory of Ocular Regenerative Medicine and Immunology, Artificial Eye Center, Seoul National University Hospital Biomedical Research Institute, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2373734
- DOI: http://doi.org/10.3346/jkms.2016.31.7.1127
Abstract
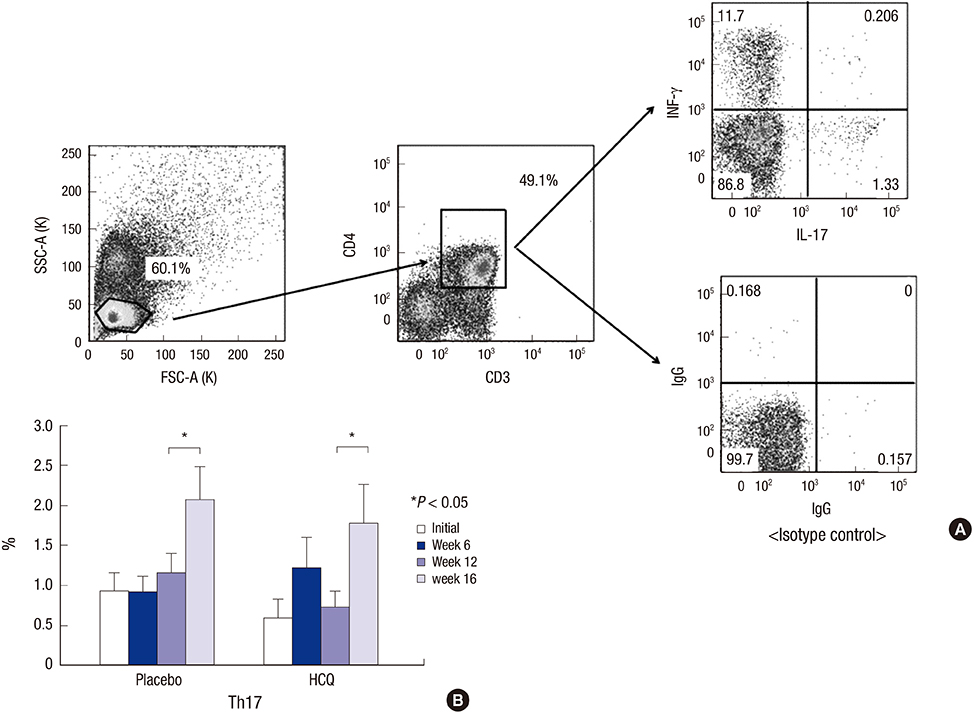
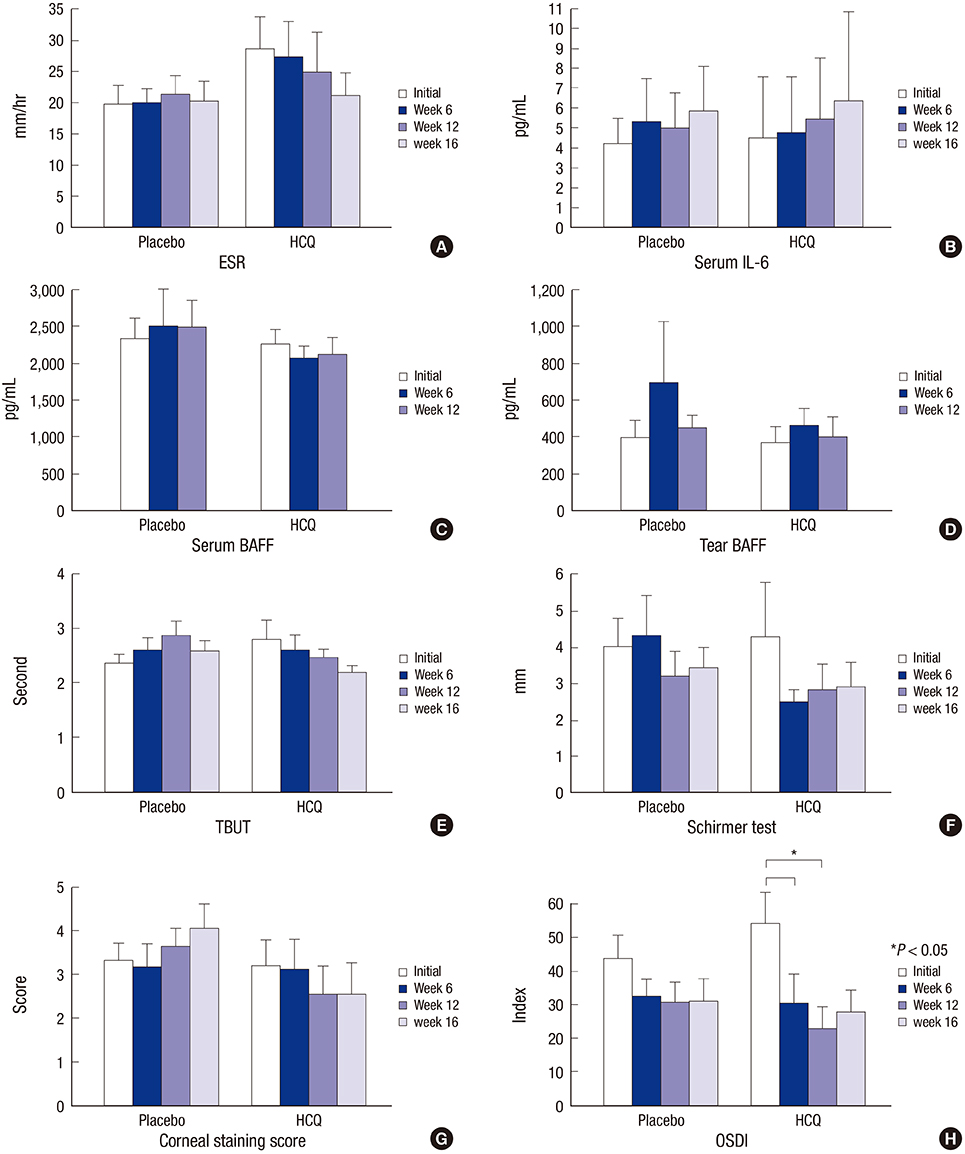
- The effect of hydroxychloroquine (HCQ) on dry eye has not been fully determined. This study aimed to compare the 12-week efficacy of HCQ medication with that of a placebo in the management of dry eye in primary Sjögren's syndrome (pSS). A double-blind, randomized control study was conducted in 39 pSS subjects from May 2011 through August 2013. pSS was diagnosed based on the classification criteria of the American-European Consensus Group. Subjects received 300 mg of HCQ or placebo once daily for 12 weeks and were evaluated at baseline, 6, and 12 weeks, with a re-visit at 16 weeks after drug discontinuance. The fluorescein staining score, Schirmer test score, tear film break-up time (TBUT), and ocular surface disease index (OSDI) were measured, and tears and blood were collected for ESR, IL-6, IL-17, B-cell activating factor (BAFF), and Th17 cell analysis. Color testing was performed and the fundus was examined to monitor HCQ complications. Twenty-six subjects completed the follow-up. The fluorescein staining score and Schirmer test score did not differ significantly. The OSDI improved with medication in the HCQ group but was not significantly different between the groups. TBUT, serum IL-6, ESR, serum and tear BAFF, and the proportion of Th17 cells did not change in either group. HCQ at 300 mg daily for 12 weeks has no apparent clinical benefit for dry eye and systemic inflammation in pSS (ClinicalTrials.gov. NCT01601028).
Keyword
MeSH Terms
-
Aged
B-Cell Activating Factor/analysis/blood
Blood Sedimentation
Double-Blind Method
Drug Administration Schedule
Dry Eye Syndromes/complications/*drug therapy
Enzyme-Linked Immunosorbent Assay
Female
Humans
Hydroxychloroquine/*therapeutic use
Interleukin-16/analysis/blood
Interleukin-17/analysis/blood
Male
Middle Aged
Placebo Effect
Prospective Studies
Sjogren's Syndrome/*complications/diagnosis
Th17 Cells/cytology/immunology
Treatment Outcome
B-Cell Activating Factor
Hydroxychloroquine
Interleukin-16
Interleukin-17
Figure
Reference
-
1. Wu CH, Hsieh SC, Lee KL, Li KJ, Lu MC, Yu CL. Pilocarpine hydrochloride for the treatment of xerostomia in patients with Sjögren’s syndrome in Taiwan--a double-blind, placebo-controlled trial. J Formos Med Assoc. 2006; 105:796–803.2. Ramos-Casals M, Tzioufas AG, Stone JH, Sisó A, Bosch X. Treatment of primary Sjögren syndrome: a systematic review. JAMA. 2010; 304:452–460.3. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61:554–558.4. Liew MS, Zhang M, Kim E, Akpek EK. Prevalence and predictors of Sjogren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012; 96:1498–1503.5. Sall KN, Cohen SM, Christensen MT, Stein JM. An evaluation of the efficacy of a cyclosporine-based dry eye therapy when used with marketed artificial tears as supportive therapy in dry eye. Eye Contact Lens. 2006; 32:21–26.6. Becker H, Pavenstaedt H, Willeke P. Emerging treatment strategies and potential therapeutic targets in primary Sjögren’s syndrome. Inflamm Allergy Drug Targets. 2010; 9:10–19.7. Tishler M, Yaron I, Shirazi I, Yaron M. Hydroxychloroquine treatment for primary Sjögren’s syndrome: its effect on salivary and serum inflammatory markers. Ann Rheum Dis. 1999; 58:253–256.8. Yavuz S, Asfuroğlu E, Bicakcigil M, Toker E. Hydroxychloroquine improves dry eye symptoms of patients with primary Sjogren’s syndrome. Rheumatol Int. 2011; 31:1045–1049.9. Kruize AA, Hené RJ, Kallenberg CG, van Bijsterveld OP, van der Heide A, Kater L, Bijlsma JW. Hydroxychloroquine treatment for primary Sjögren’s syndrome: a two year double blind crossover trial. Ann Rheum Dis. 1993; 52:360–364.10. Gottenberg JE, Ravaud P, Puéchal X, Le Guern V, Sibilia J, Goeb V, Larroche C, Dubost JJ, Rist S, Saraux A, et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA. 2014; 312:249–258.11. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiødt M, Umehara H, Vivino F, Zhao Y, et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012; 64:475–487.12. Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995; 21:221–232.13. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000; 118:615–621.14. Acera A, Rocha G, Vecino E, Lema I, Durán JA. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008; 40:315–321.15. Chung JK, Kim MK, Wee WR. Prognostic factors for the clinical severity of keratoconjunctivitis sicca in patients with Sjogren’s syndrome. Br J Ophthalmol. 2012; 96:240–245.16. da Silva JC, Mariz HA, da Rocha LF Jr, de Oliveira PS, Dantas AT, Duarte AL, da Rocha Pitta I, Galdino SL, da Rocha Pitta MG. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (Sao Paulo). 2013; 68:766–771.17. Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011; 186:4794–4804.18. Seillet C, Laffont S, Trémollières F, Rouquié N, Ribot C, Arnal JF, Douin-Echinard V, Gourdy P, Guéry JC. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012; 119:454–464.19. Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012; 181:8–18.20. Mills KH. TLR9 turns the tide on Treg cells. Immunity. 2008; 29:518–520.21. Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjögren’s syndrome immunopathogenesis. Am J Pathol. 2009; 175:1167–1177.22. Oh JY, Kim MK, Choi HJ, Ko JH, Kang EJ, Lee HJ, Wee WR, Lee JH. Investigating the relationship between serum interleukin-17 levels and systemic immune-mediated disease in patients with dry eye syndrome. Korean J Ophthalmol. 2011; 25:73–76.23. Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: findings in humans and mice. Arthritis Rheum. 2008; 58:734–743.24. Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH. Interleukin-17 in various ocular surface inflammatory diseases. J Korean Med Sci. 2011; 26:938–944.25. Moisini I, Davidson A. BAFF: a local and systemic target in autoimmune diseases. Clin Exp Immunol. 2009; 158:155–163.26. Roescher N, Tak PP, Illei GG. Cytokines in Sjogren’s syndrome: potential therapeutic targets. Ann Rheum Dis. 2010; 69:945–948.27. Mumcu G, Biçakçigil M, Yilmaz N, Ozay H, Karaçayli U, Cimilli H, Yavuz S. Salivary and serum B-cell activating factor (BAFF) levels after hydroxychloroquine treatment in primary Sjögren’s syndrome. Oral Health Prev Dent. 2013; 11:229–234.28. Fox RI, Dixon R, Guarrasi V, Krubel S. Treatment of primary Sjögren’s syndrome with hydroxychloroquine: a retrospective, open-label study. Lupus. 1996; 5:Suppl 1. S31–6.29. Rihl M, Ulbricht K, Schmidt RE, Witte T. Treatment of sicca symptoms with hydroxychloroquine in patients with Sjogren’s syndrome. Rheumatology (Oxford). 2009; 48:796–799.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Treatment with Steroid and Hydrochloroquine of Thrombocytopenia in Primary Sjögren's Syndrome
- Correlation between Tear Osmolarity and Other Ocular Surface Parameters in Primary Sjögren's Syndrome
- Longitudinal Changes of the European League Against Rheumatism Sjögren's Syndrome Patient Reported Index in Korean Patients with Primary Sjögren's Syndrome
- A Case of Distal Renal Tubular Acidosis with Sjögren's Syndrome Presenting as Hypokalemic Paralysis
- A Case of Sjögren-Larsson Syndrome