Ann Lab Med.
2016 Jul;36(4):362-366. 10.3343/alm.2016.36.4.362.
Effect of Irradiation on Microparticles in Red Blood Cell Concentrates
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, Korea University, Seoul, Korea. malarim@korea.ac.kr
- 2The Armed Forces Daejeon Hospital, Daejeon, Korea.
- KMID: 2373557
- DOI: http://doi.org/10.3343/alm.2016.36.4.362
Abstract
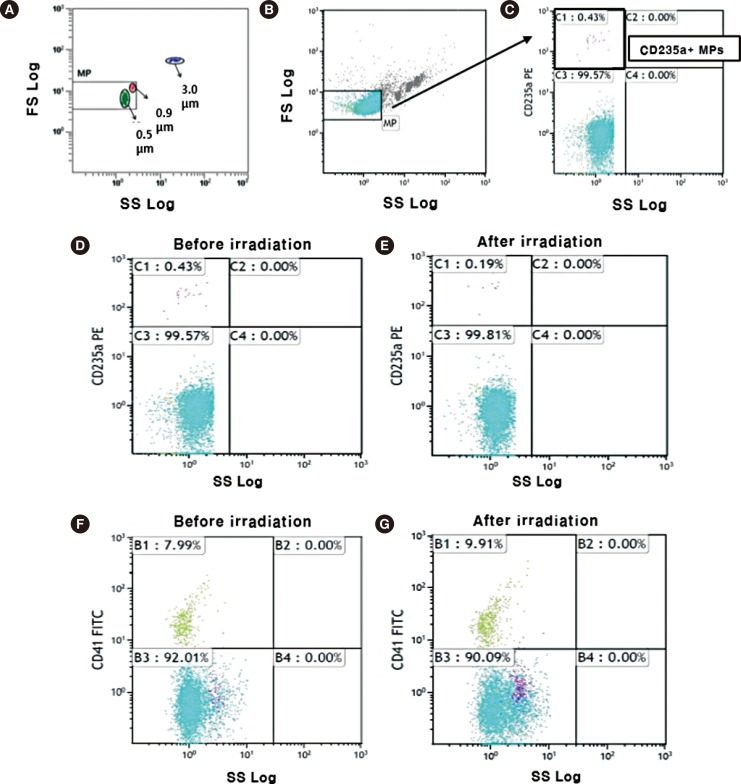
- Changes in microparticles (MP) from red blood cell (RBC) concentrates in the context of irradiation have not been investigated. The aim of this study was to evaluate how irradiation affects the number of MPs within transfusion components. Twenty RBC concentrates, within 14 days after donation, were exposed to gamma rays (dose rate: 25 cGy) from a cesium-137 irradiator. Flow cytometry was used to determine the numbers of MPs derived from RBC concentrates before and 24 hr after irradiation. The mean number of MPs (±standard deviation) in RBC concentrates was 21.9×10(9)/L (±22.7×10(9)/L), and the total number of MPs ranged from 2.6×10(9)/L to 96.9×10(9)/L. The mean number of MPs increased to 22.6×10(9)/L (±31.6×10(9)/L) after irradiation. Before irradiation, the CD41-positive and CD235a-positive MPs constituted 9.5% (1.0×10(9)/L) and 2.2% (263×10(6)/L) of total MPs, respectively. After irradiation, CD41-positive MPs increased to 12.1% (1.5×10(9)/L) (P=0.014), but the CD235a-positive MPs decreased to 2.0% (214×10(6)/L) of the total MPs (P=0.369). Irradiation increases the number of CD41-positive MPs within RBC concentrates, suggesting the irradiation of RBC concentrates could be associated with thrombotic risk of circulating blood through the numerical change.
Keyword
MeSH Terms
-
Cell-Derived Microparticles/chemistry/*metabolism/radiation effects
Erythrocytes/*cytology/radiation effects
Flow Cytometry
Gamma Rays
Humans
Membrane Glycoproteins/metabolism
Metalloendopeptidases/metabolism
Platelet Membrane Glycoprotein IIb/metabolism
Membrane Glycoproteins
Metalloendopeptidases
Platelet Membrane Glycoprotein IIb
Figure
Reference
-
1. Ayers L, Kohler M, Harrison P, Sargent I, Dragovic R, Schaap M, et al. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb Res. 2011; 127:370–377. PMID: 21257195.
Article2. Barteneva NS, Fasler-Kan E, Bernimoulin M, Stern JN, Ponomarev ED, Duckett L, et al. Circulating microparticles: square the circle. BMC Cell Biol. 2013; 14:23. PMID: 23607880.
Article3. Jayachandran M, Litwiller RD, Owen WG, Heit JA, Behrenbeck T, Mulvagh SL, et al. Characterization of blood borne microparticles as markers of premature coronary calcification in newly menopausal women. Am J Physiol Heart Circ Physiol. 2008; 295:H931–H938. PMID: 18621859.
Article4. Jung KH, Chu K, Lee ST, Park HK, Bahn JJ, Kim DH, et al. Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann Neurol. 2009; 66:191–199. PMID: 19743467.
Article5. Canellini G, Rubin O, Delobel J, Crettaz D, Lion N, Tissot JD. Red blood cell microparticles and blood group antigens: an analysis by flow cytometry. Blood Transfus. 2012; 10(S2):S39–S45. PMID: 22890266.6. Chan KS, Sparrow RL. Microparticle profile and procoagulant activity of fresh-frozen plasma is affected by whole blood leukoreduction rather than 24-hour room temperature hold. Transfusion. 2014; 54:1935–1944. PMID: 24635475.
Article7. Lawrie AS, Harrison P, Cardigan RA, Mackie IJ. The characterization and impact of microparticles on haemostasis within fresh-frozen plasma. Vox Sang. 2008; 95:197–204. PMID: 19121184.
Article8. Keuren JF, Magdeleyns EJ, Govers-Riemslag JW, Lindhout T, Curvers J. Effects of storage-induced platelet microparticles on the initiation and propagation phase of blood coagulation. Br J Haematol. 2006; 134:307–313. PMID: 16848773.
Article9. Robert S, Poncelet P, Lacroix R, Arnaud L, Giraudo L, Hauchard A, et al. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies. J Thromb Haemost. 2009; 7:190–197. PMID: 18983485.
Article10. Liu C, Zhao W, Christ GJ, Gladwin MT, Kim-Shapiro DB. Nitric oxide scavenging by red cell microparticles. Free Radic Biol Med. 2013; 65:1164–1173. PMID: 24051181.
Article11. Kriebardis A, Antonelou M, Stamoulis K, Papassideri I. Cell-derived microparticles in stored blood products: innocent-bystanders or effective mediators of post-transfusion reactions. Blood Transfus. 2012; 10(S2):S25–S38. PMID: 22890265.12. Rubin O, Delobel J, Prudent M, Lion N, Kohl K, Tucker EI, et al. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013; 53:1744–1754. PMID: 23228139.
Article13. Mezouar S, Mege D, Darbousset R, Farge D, Debourdeau P, Dignat-George F, et al. Involvement of platelet-derived microparticles in tumor progression and thrombosis. Semin Oncol. 2014; 41:346–358. PMID: 25023350.
Article14. Agouti I, Cointe S, Robert S, Judicone C, Loundou A, Driss F, et al. Platelet and not erythrocyte microparticles are procoagulant in transfused thalassaemia major patients. Br J Haematol. 2015; 171:615–624. PMID: 26205481.
Article15. Nebor D, Bowers A, Connes P, Hardy-Dessources MD, Knight-Madden J, Cumming V, et al. Plasma concentration of platelet-derived microparticles is related to painful vaso-occlusive phenotype severity in sickle cell anemia. PLoS One. 2014; 9:e87243. PMID: 24475257.
Article16. Goubran HA, Burnouf T, Stakiw J, Seghatchian J. Platelet microparticle: a sensitive physiological "fine tuning" balancing factor in health and disease. Transfus Apher Sci. 2015; 52:12–18. PMID: 25599988.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidence of Red Blood Cell Alloantibody Formation after Platelet Concentrate Transfusions
- Changes in the Physical Properties of Irradiated Red Blood Cells
- Effect of 137Cs Whole Body Irradiation on the Peripheral Blood
- Current Status of Irradiated Blood Components and Blood Irradiators in Korean Medical Institutes
- Preparation of Collagen Modified Hyaluronan Microparticles as Antibiotics Carrier