Ann Lab Med.
2016 Mar;36(2):154-161. 10.3343/alm.2016.36.2.154.
Survey of Clinical Laboratory Practices for 2015 Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 2Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. jeongho@yuhs.ac
- 3Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Laboratory Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 6Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 7Department of Laboratory Medicine, Catholic Kwandong University College of Medicine, Incheon, Korea.
- KMID: 2373515
- DOI: http://doi.org/10.3343/alm.2016.36.2.154
Abstract
- BACKGROUND
It is crucial to understand the current status of clinical laboratory practices for the largest outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infections in the Republic of Korea to be well prepared for future emerging infectious diseases.
METHODS
We conducted a survey of 49 clinical laboratories in medical institutions and referral medical laboratories. A short questionnaire to survey clinical laboratory practices relating to MERS-CoV diagnostic testing was sent by email to the directors and clinical pathologists in charge of the clinical laboratories performing MERS-CoV testing. The survey focused on testing volume, reporting of results, resources, and laboratory safety.
RESULTS
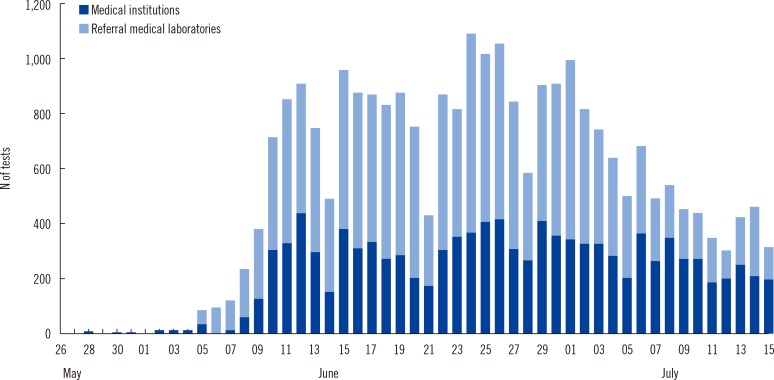
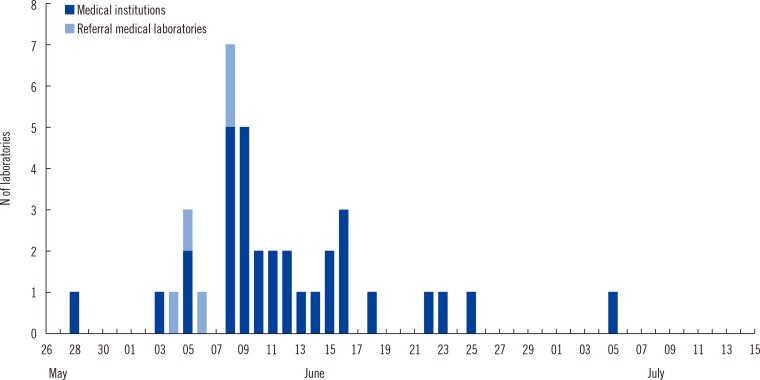
A total of 40 clinical laboratories responded to the survey. A total of 27,009 MERS-CoV real-time reverse transcription PCR (rRT-PCR) tests were performed. Most of the specimens were sputum (73.5%). The median turnaround time (TAT) was 5.29 hr (first and third quartile, 4.11 and 7.48 hr) in 26 medical institutions. The median TAT of more than a half of the laboratories (57.7%) was less than 6 hr. Many laboratories were able to perform tests throughout the whole week. Laboratory biosafety preparedness included class II biosafety cabinets (100%); separated pre-PCR, PCR, and post-PCR rooms (88.6%); negative pressure pretreatment rooms (48.6%); and negative pressure sputum collection rooms (20.0%).
CONCLUSIONS
Clinical laboratories were able to quickly expand their diagnostic capacity in response to the 2015 MERS-CoV outbreak. Our results show that clinical laboratories play an important role in the maintenance and enhancement of laboratory response in preparation for future emerging infections.
Keyword
MeSH Terms
-
Clinical Laboratory Services/*standards
Clinical Laboratory Techniques/instrumentation/methods
Coronavirus Infections/*diagnosis/epidemiology/virology
Disease Outbreaks
Humans
Middle East Respiratory Syndrome Coronavirus/genetics/isolation & purification
RNA, Viral/analysis
Real-Time Polymerase Chain Reaction
Republic of Korea/epidemiology
Sputum/virology
Surveys and Questionnaires
RNA, Viral
Figure
Cited by 3 articles
-
Surveillance of Coronavirus Disease 2019 (COVID-19) Testing in Clinical Laboratories in Korea
Hee Jae Huh, Ki Ho Hong, Taek Soo Kim, Sang Hoon Song, Kyoung Ho Roh, Hyukmin Lee, Gye Cheol Kwon
Ann Lab Med. 2021;41(2):225-229. doi: 10.3343/alm.2021.41.2.225.Quality of Ribonucleic Acid Extraction for Real-Time Reverse Transcription-PCR (rRT-PCR) of SARS-CoV-2: Importance of Internal Control Monitoring
Yeon Joo Lee, Youngeun Lim, Kyu Wha Hur, Heungsup Sung, Mi-Na Kim
Ann Lab Med. 2020;40(6):490-492. doi: 10.3343/alm.2020.40.6.490.Considering Revision the Criteria for Patients under Investigations for MERS-CoV Infections: Diarrhea or Not
Mi-Na Kim, Eui-Chong Kim
J Korean Med Sci. 2018;33(53):. doi: 10.3346/jkms.2018.33.e344.
Reference
-
1. Cowling BJ, Park M, Fang VJ, Wu P, Leung GM, Wu JT. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 2015; 20:7–13. PMID: 26132767.
Article2. Hayden RT, Wick MT, Rodriguez AB, Caliendo AM, Mitchell MJ, Ginocchio CC. A survey-based assessment of United States clinical laboratory response to the 2009 H1N1 influenza outbreak. Arch Pathol Lab Med. 2010; 134:1671–1678. PMID: 21043821.
Article3. Sullivan SJ, Jacobson RM, Dowdle WR, Poland GA. 2009 H1N1 influenza. Mayo Clin Proc. 2010; 85:64–76. PMID: 20007905.
Article4. Crawford JM, Stallone R, Zhang F, Gerolimatos M, Korologos DD, Sweetapple C, et al. Laboratory surge response to pandemic (H1N1) 2009 outbreak, New York City metropolitan area, USA. Emerg Infect Dis. 2010; 16:8–13. PMID: 20031036.
Article5. Lautenbach E, Saint S, Henderson DK, Harris AD. Initial response of health care institutions to emergence of H1N1 influenza: experiences, obstacles, and perceived future needs. Clin Infect Dis. 2010; 50:523–527. PMID: 20064038.
Article6. Lee WG, Kwak YS, Lee DH, Hwang YS, Lee KN. Clinical pathology laboratory inspection and accreditation in Korea I: Development of the system and its trial. Korean J Clin Pathol. 2001; 21:86–92.7. Oboho IK, Tomczyk SM, Al-Asmari AM, Banjar AA, Al-Mugti H, Aloraini MS, et al. 2014 MERS-CoV outbreak in Jeddah--a link to health care facilities. N Engl J Med. 2015; 372:846–854. PMID: 25714162.8. Tartari E, Allegranzi B, Ang B, Calleja N, Collignon P, Hopman J, et al. Preparedness of institutions around the world for managing patients with Ebola virus disease: an infection control readiness checklist. Antimicrob Resist Infect Control. 2015; 4:22. PMID: 26056563.
Article9. Breban R, Riou J, Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013; 382:694–699. PMID: 23831141.
Article10. Pereyaslov D, Rosin P, Palm D, Zeller H, Gross D, Brown CS, et al. Laboratory capability and surveillance testing for Middle East respiratory syndrome coronavirus infection in the WHO European Region, June 2013. Euro Surveill. 2014; 19:20923. PMID: 25323078.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Unexpected Outbreak of Middle East Respiratory Syndrome Coronavirus Infection in the Republic of Korea, 2015
- The Same Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) yet Different Outbreak Patterns and Public Health Impacts on the Far East Expert Opinion from the Rapid Response Team of the Republic of Korea
- Costly Lessons From the 2015 Middle East Respiratory Syndrome Coronavirus Outbreak in Korea
- Middle East respiratory syndrome: what we learned from the 2015 outbreak in the Republic of Korea
- The Korean Middle East Respiratory Syndrome Coronavirus Outbreak and Our Responsibility to the Global Scientific Community