Ann Lab Med.
2016 Jan;36(1):82-84. 10.3343/alm.2016.36.1.82.
Bone Marrow Chimerism Detection Using Next Generation Sequencing Based on Single Nucleotide Polymorphisms Following Liver Transplantation: Comparison With Short Tandem Repeat-PCR
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Yonsei University College of Medicine, Seoul, Korea. cjr0606@yuhs.ac
- 2Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Surgery and Research Institute for Transplantation, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2373506
- DOI: http://doi.org/10.3343/alm.2016.36.1.82
Abstract
- No abstract available.
MeSH Terms
-
Adult
Bone Marrow/*pathology
Fatal Outcome
Graft vs Host Disease/etiology
High-Throughput Nucleotide Sequencing
Humans
Liver Cirrhosis/pathology/*therapy
*Liver Transplantation/adverse effects
Microsatellite Repeats
Middle Aged
Polymerase Chain Reaction
*Polymorphism, Single Nucleotide
Transplantation Chimera/*genetics
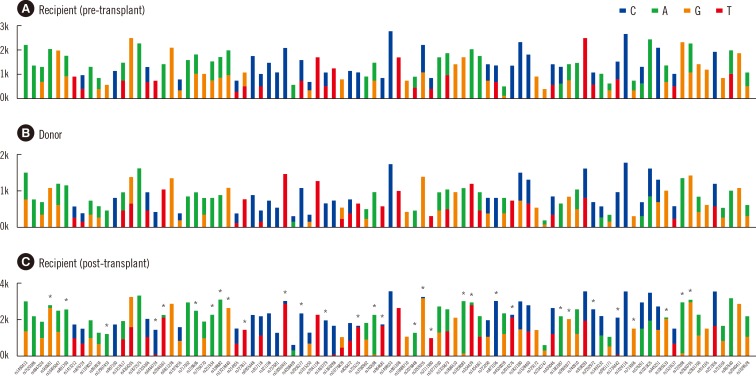
Figure
Reference
-
1. Smith DM, Agura E, Netto G, Collins R, Levy M, Goldstein R, et al. Liver transplant-associated graft-versus-host disease. Transplantation. 2003; 75:118–126. PMID: 12544883.2. Alexander SI, Smith N, Hu M, Verran D, Shun A, Dorney S, et al. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med. 2008; 358:369–374. PMID: 18216357.3. Ayala R, Grande S, Albizua E, Crooke A, Meneu JC, Moreno A, et al. Long-term follow-up of donor chimerism and tolerance after human liver transplantation. Liver Transpl. 2009; 15:581–591. PMID: 19479801.4. Pollack MS, Speeg KV, Callander NS, Freytes CO, Espinoza AA, Esterl RM, et al. Severe, late-onset graft-versus-host disease in a liver transplant recipient documented by chimerism analysis. Hum Immunol. 2005; 66:28–31. PMID: 15620459.5. Nollet F, Billiet J, Selleslag D, Criel A. Standardisation of multiplex fluorescent short tandem repeat analysis for chimerism testing. Bone Marrow Transplant. 2001; 28:511–518. PMID: 11593326.6. Smith EP. Hematologic disorders after solid organ transplantation. Hematology Am Soc Hematol Educ Program. 2010; 2010:281–286. PMID: 21239807.7. Domiati-Saad R, Klintmalm GB, Netto G, Agura ED, Chinnakotla S, Smith DM. Acute graft versus host disease after liver transplantation: patterns of lymphocyte chimerism. Am J Transplant. 2005; 5:2968–2973. PMID: 16303012.8. Thiede C, Florek M, Bornhäuser M, Ritter M, Mohr B, Brendel C, et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant. 1999; 23:1055–1060. PMID: 10373073.9. Templeton JE, Brotherton PM, Llamas B, Soubrier J, Haak W, Cooper A, et al. DNA capture and next-generation sequencing can recover whole mitochondrial genomes from highly degraded samples for human identification. Investig Genet. 2013; 4:26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chimerism by Analysis of PCR of Highly Polymorphic Variable Number of Tandem Repeat (VNTR) DNA Sequences in Human Genome; The Graft Versus Host Disease (GVHD) and Relapse of Leukemia after Allogeneic Bone Marrow Transplantation
- The Discrimination Power and Effectiveness of 3 Kinds of LTR Primers in the VNTR-PCR for Evaluation of the Engraftment of Allogeneic Peripheral Blood Stem Cells Transplantation
- Comparison of single nucleotide polymorphisms and short tandem repeats as markers for differentiating between donors and recipients in solid organ transplantation
- Clinical Utility of Chimerism Status Assessed by Lineage-Specific Short Tandem Repeat Analysis: Experience from Four Cases of Allogeneic Stem Cell Transplantation
- Analysis of PCR-Based VNTR Markers to Evaluate Engraftment Status after Bone Marrow Transplantation