Ann Lab Med.
2015 Sep;35(5):535-539. 10.3343/alm.2015.35.5.535.
CYP21A2 Mutation Analysis in Korean Patients With Congenital Adrenal Hyperplasia Using Complementary Methods: Sequencing After Long-Range PCR and Restriction Fragment Length Polymorphism Analysis With Multiple Ligation-Dependent Probe Amplification Assay
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. nayadoo@hanmail.net
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- KMID: 2369771
- DOI: http://doi.org/10.3343/alm.2015.35.5.535
Abstract
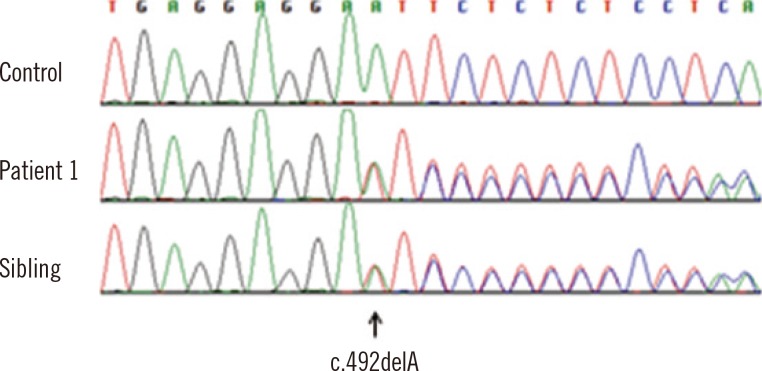
- CYP21A2 mutation analysis of congenital adrenal hyperplasia (CAH) is challenging because of the genomic presence of a homologous CYP21A2 pseudogene and the significant incidence of pseudogene conversion and large deletions. The objective of this study was to accurately analyze the CYP21A2 genotype in Korean CAH patients using a combination of complementary methods. Long-range PCR and restriction fragment length polymorphism analyses were performed to confirm valid amplification of CYP21A2 and to detect large gene conversions and deletions before direct sequencing. Multiple ligation-dependent probe amplification (MLPA) analysis was conducted concurrently in 14 CAH-suspected patients and six family members of three patients. We identified 27 CYP21A2 mutant alleles in 14 CAH-suspected patients. The c.293-13A>G (or c.293-13C>G) was the most common mutation, and p.Ile173Asn was the second, identified in 25% and 17.9% of alleles, respectively. A novel frame-shift mutation of c.492delA (p.Glu 164Aspfs*24) was detected. Large deletions were detected by MLPA in 10.7% of the alleles. Mutation studies of the six familial members for three of the patients aided in the identification of haplotypes. In summary, we successfully identified CYP21A2 mutations using both long-range PCR and sequencing and dosage analyses. Our data correspond relatively well with the previously reported mutation spectrum analysis.
Keyword
MeSH Terms
Figure
Reference
-
1. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000; 21:245–291. PMID: 10857554.
Article2. Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:4133–4160. PMID: 20823466.
Article3. White PC, New MI, Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986; 83:5111–5115. PMID: 3487786.
Article4. Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003; 349:776–788. PMID: 12930931.
Article5. Lee HH. Variants of the CYP21A2 and CYP21A1P genes in congenital adrenal hyperplasia. Clin Chim Acta. 2013; 418:37–44. PMID: 23313747.
Article6. Lee HH, Lee YJ, Chao MC. Comparing the Southern blot method and polymerase chain reaction product analysis for chimeric RCCX detection in CYP21A2 deficiency. Anal Biochem. 2010; 399:293–298. PMID: 19961824.
Article7. Lee HH, Lee YJ, Lin CY. PCR-based detection of the CYP21 deletion and TNXA/TNXB hybrid in the RCCX module. Genomics. 2004; 83:944–950. PMID: 15081125.
Article8. New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, et al. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 2013; 110:2611–2616. PMID: 23359698.
Article9. Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab. 2000; 85:1059–1065. PMID: 10720040.
Article10. Lee HH, Lee YJ, Wang YM, Chao HT, Niu DM, Chao MC, et al. Low frequency of the CYP21A2 deletion in ethnic Chinese (Taiwanese) patients with 21-hydroxylase deficiency. Mol Genet Metab. 2008; 93:450–457. PMID: 18039588.
Article11. Wedell A. Molecular genetics of congenital adrenal hyperplasia (21-hydroxylase deficiency): implications for diagnosis, prognosis and treatment. Acta Paediatr. 1998; 87:159–164. PMID: 9512201.
Article12. Marino R, Ramirez P, Galeano J, Perez Garrido N, Rocco C, Ciaccio M, et al. Steroid 21-hydroxylase gene mutational spectrum in 454 Argentinean patients: genotype-phenotype correlation in a large cohort of patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2011; 75:427–435. PMID: 21609351.
Article13. Stikkelbroeck NM, Hoefsloot LH, de Wijs IJ, Otten BJ, Hermus AR, Sistermans EA. CYP21 gene mutation analysis in 198 patients with 21-hydroxylase deficiency in The Netherlands: six novel mutations and a specific cluster of four mutations. J Clin Endocrinol Metab. 2003; 88:3852–3859. PMID: 12915679.
Article14. Ma D, Chen Y, Sun Y, Yang B, Cheng J, Huang M, et al. Molecular analysis of the CYP21A2 gene in Chinese patients with steroid 21-hydroxylase deficiency. Clin Biochem. 2014; 47:455–463. PMID: 24503005.
Article15. Yoo Y, Chang MS, Lee J, Cho SY, Park SW, Jin D-K, et al. Genotype-phenotype correlation in 27 pediatric patients in congenital adrenal hyperplasia due to 21-hydroxylase deficiency in a single center. Ann Pediatr Endocrinol Metab. 2013; 18:128. PMID: 24904866.
Article16. Jang JH, Jin DK, Kim JH, Tan HK, Kim JW, Lee SY, et al. Multiplex ligation-dependent probe amplification assay for diagnosis of congenital adrenal hyperplasia. Ann Clin Lab Sci. 2011; 41:44–47. PMID: 21325254.17. Choi JH, Jin HY, Lee BH, Ko JM, Lee JJ, Kim GH, et al. Clinical phenotype and mutation spectrum of the CYP21A2 gene in patients with steroid 21-hydroxylase deficiency. Exp Clin Endocrinol Diabetes. 2012; 120:23–27. PMID: 22020670.
Article18. Xu Z, Chen W, Merke DP, McDonnell NB. Comprehensive mutation analysis of the CYP21A2 gene: an efficient multistep approach to the molecular diagnosis of congenital adrenal hyperplasia. J Mol Diagn. 2013; 15:745–753. PMID: 24071710.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Genetic Studies on the Human CYP21A2 Gene (1)
- A Molecular Genetic Study with EcoRII Restriction Enzyme on the Steroidogenic Acute Regulatory Protein (StAR) Gene
- Direct detection of hemophilia B F9 gene mutation using multiplex PCR and conformation sensitive gel electrophoresis
- Compound heterozygosity for a whole gene deletion and p.R124C mutation in CYP21A2 causing nonclassic congenital adrenal hyperplasia
- Absence of TaqI Polymorphism in Exons of Complement Component C9 Gene in Koreans