Effect of Accreditation on Accuracy of Diagnostic Tests in Medical Laboratories
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea. lywmd@schmc.ac.kr cecilia@schmc.ac.kr
- 2Department of Laboratory Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 3Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 6Clinical Trial Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2369746
- DOI: http://doi.org/10.3343/alm.2017.37.3.213
Abstract
- BACKGROUND
Medical laboratories play a central role in health care. Many laboratories are taking a more focused and stringent approach to quality system management. In Korea, laboratory standardization efforts undertaken by the Korean Laboratory Accreditation Program (KLAP) and the Korean External Quality Assessment Scheme (KEQAS) may have facilitated an improvement in laboratory performance, but there are no fundamental studies demonstrating that laboratory standardization is effective. We analyzed the results of the KEQAS to identify significant differences between laboratories with or without KLAP and to determine the impact of laboratory standardization on the accuracy of diagnostic tests.
METHODS
We analyzed KEQAS participant data on clinical chemistry tests such as albumin, ALT, AST, and glucose from 2010 to 2013. As a statistical parameter to assess performance bias between laboratories, we compared 4-yr variance index score (VIS) between the two groups with or without KLAP.
RESULTS
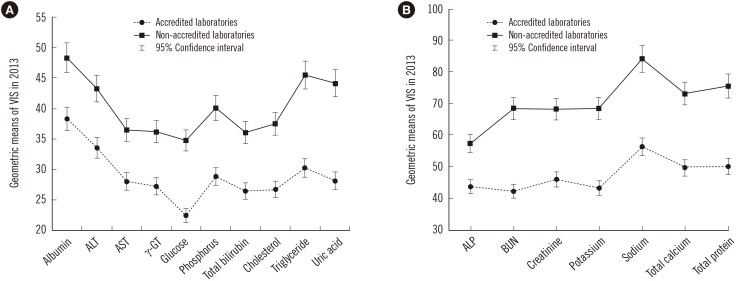
Compared with the group without KLAP, the group with KLAP exhibited significantly lower geometric means of 4-yr VIS for all clinical chemistry tests (P<0.0001); this difference justified a high level of confidence in standardized services provided by accredited laboratories. Confidence intervals for the mean of each test in the two groups (accredited and non-accredited) did not overlap, suggesting that the means of the groups are significantly different.
CONCLUSIONS
These results confirmed that practice standardization is strongly associated with the accuracy of test results. Our study emphasizes the necessity of establishing a system for standardization of diagnostic testing.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Korean Clinical Laboratory Accreditation Program Quality Standards for Laboratory Management: Identifying a Compliance Gap with World Health Organization Quality System Essentials
Byung Ryul Jeon, Chiho Yoon, Mi-Ae Jang, Sung Ran Cho, Sollip Kim, You Kyoung Lee
Lab Med Online. 2020;10(2):152-159. doi: 10.3343/lmo.2020.10.2.152.Effect of the Standardization of Diagnostic Tests on the Prevalence of Diabetes Mellitus and Impaired Fasting Glucose
Bora Lee, Ji Sung Lee, Yong-Wha Lee, Mi-Ae Jang, Junghan Song, Jeong-Ho Kim, Wee Gyo Lee, Won-Ki Min, Juneyoung Lee, You Kyoung Lee
J Korean Med Sci. 2018;33(10):. doi: 10.3346/jkms.2018.33.e81.Comprehensive Laboratory Data Analysis to Predict the Clinical Severity of Coronavirus Disease 2019 in 1,952 Patients in Daegu, Korea
Eun-Hyung Yoo, Soon Hee Chang, Do-Young Song, Chae Hoon Lee, Gyu Young Cheong, Sunggyun Park, Jae Hee Lee, Sooin Lee, Sang-Gyu Kwak, Chang-Ho Jeon, Kyung Eun Song
Ann Lab Med. 2022;42(1):24-35. doi: 10.3343/alm.2022.42.1.24.국가 간암 검진 프로그램 수행 임상검사실의 품질 평가
Hyeseong Ryu, Sollip Kim, Hyeongsu Kim, Hye Ryun Lee, Yeo-Min Yun, Ho Jin Jeong, Minje Han, Myeong Hee Kim, Tae-Hyun Um, You Kyoung Lee, Byung Ryul Jeon, Kunsei Lee, Sail Chun
Lab Med Online. 2025;15(2):154-158. doi: 10.47429/lmo.2025.15.2.154.
Reference
-
1. Forsman RW. Why is the laboratory an afterthought for managed care organizations? Clin Chem. 1996; 42:813–816. PMID: 8653920.2. Burnett D. ISO 15189:2003--quality management, evaluation and continual improvement. Clin Chem Lab Med. 2006; 44:733–739. PMID: 16729862.3. McPherson RA, Pincus MR, editors. Henry's clinical diagnosis and management by laboratory methods. 23th ed. St. Louis, MO: Elsevier;2017. p. 2–5.4. Medicare, Medicaid and CLIA programs; regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA)--HCFA. Final rule with comment period. Fed Regist. 1992; 57:7002–7186. PMID: 10170937.5. Cho HI. Twenty-years of experiences in external quality assurance in Korea. Southeast Asian J Trop Med Public Health. 1999; 30(S3):32–38.6. Shin BM, Chae SL, Min WK, Lee WG, Lim YA, Lee DH, et al. The implementation and effects of a clinical laboratory accreditation program in Korea from 1999 to 2006. Korean J Lab Med. 2009; 29:163–170. PMID: 19411785.7. Jun SH, Song J. Annual Report on the External Quality Assessment Scheme in Clinical Chemistry in Korea (2013). J Lab Med Qual Assur. 2014; 36:113–121.8. Whitehead TP, Woodford FP. External quality assessment of clinical laboratories in the United Kingdom. J Clin Pathol. 1981; 34:947–957. PMID: 7024326.9. Huisman W, Horvath AR, Burnett D, Blaton V, Czikkely R, Jansen RT, et al. Accreditation of medical laboratories in the European Union. Clin Chem Lab Med. 2007; 45:268–275. PMID: 17311523.10. Guzel O, Guner EI. ISO 15189 accreditation: Requirements for quality and competence of medical laboratories, experience of a laboratory I. Clin Biochem. 2009; 42:274–278. PMID: 19863920.11. Kibet E, Moloo Z, Ojwang PJ, Sayed S, Mbuthia A, Adam RD. Measurement of improvement achieved by participation in international laboratory accreditation in sub-Saharan Africa: the Aga Khan University Hospital Nairobi experience. Am J Clin Pathol. 2014; 141:188–195. PMID: 24436265.12. Gershy-Damet GM, Rotz P, Cross D, Belabbes el H, Cham F, Ndihokubwayo JB, et al. The World Health Organization African region laboratory accreditation process: improving the quality of laboratory systems in the African region. Am J Clin Pathol. 2010; 134:393–400. PMID: 20716795.13. Sciacovelli L, Plebani M. The IFCC Working Group on laboratory errors and patient safety. Clin Chim Acta. 2009; 404:79–85. PMID: 19328194.14. Jamtsho R, Nuchpramool W. Implementation of External Quality Assessment Scheme in clinical chemistry for district laboratories in Bhutan. Indian J Clin Biochem. 2012; 27:300–305. PMID: 26405392.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is accreditation in medical education in Korea an opportunity or a burden?
- Meta-Analysis of Diagnostic Test Accuracy
- International Organization for Standardization (ISO) 15189
- Overview of the Process of Conducting Meta-analyses of the Diagnostic Test Accuracy
- Analysis on the Performance and Tasks of Accreditation System for Medical Colleges