J Pathol Transl Med.
2017 Jan;51(1):56-68. 10.4132/jptm.2016.09.17.
Size of Non-lepidic Invasive Pattern Predicts Recurrence in Pulmonary Mucinous Adenocarcinoma: Morphologic Analysis of 188 Resected Cases with Reappraisal of Invasion Criteria
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hanjho@skku.edu
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Thoracic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2367681
- DOI: http://doi.org/10.4132/jptm.2016.09.17
Abstract
- BACKGROUND
We reviewed a series of 188 resected pulmonary mucinous adenocarcinomas (MAs) to clarify the prognostic significance of lepidic and non-lepidic patterns.
METHODS
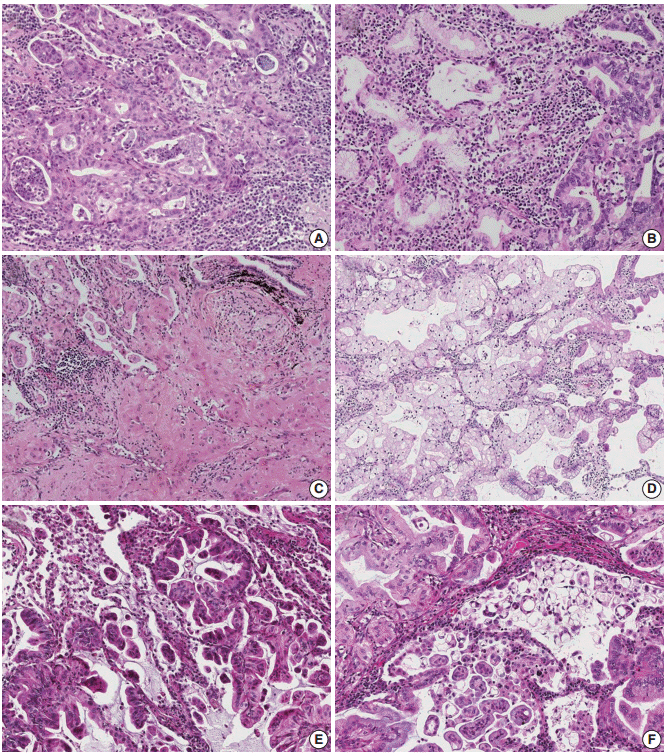
Non-lepidic patterns were divided into bland, non-distorted acini with uncertain invasiveness (pattern 1), unequivocal invasion into stroma (pattern 2), or invasion into alveolar spaces (pattern 3).
RESULTS
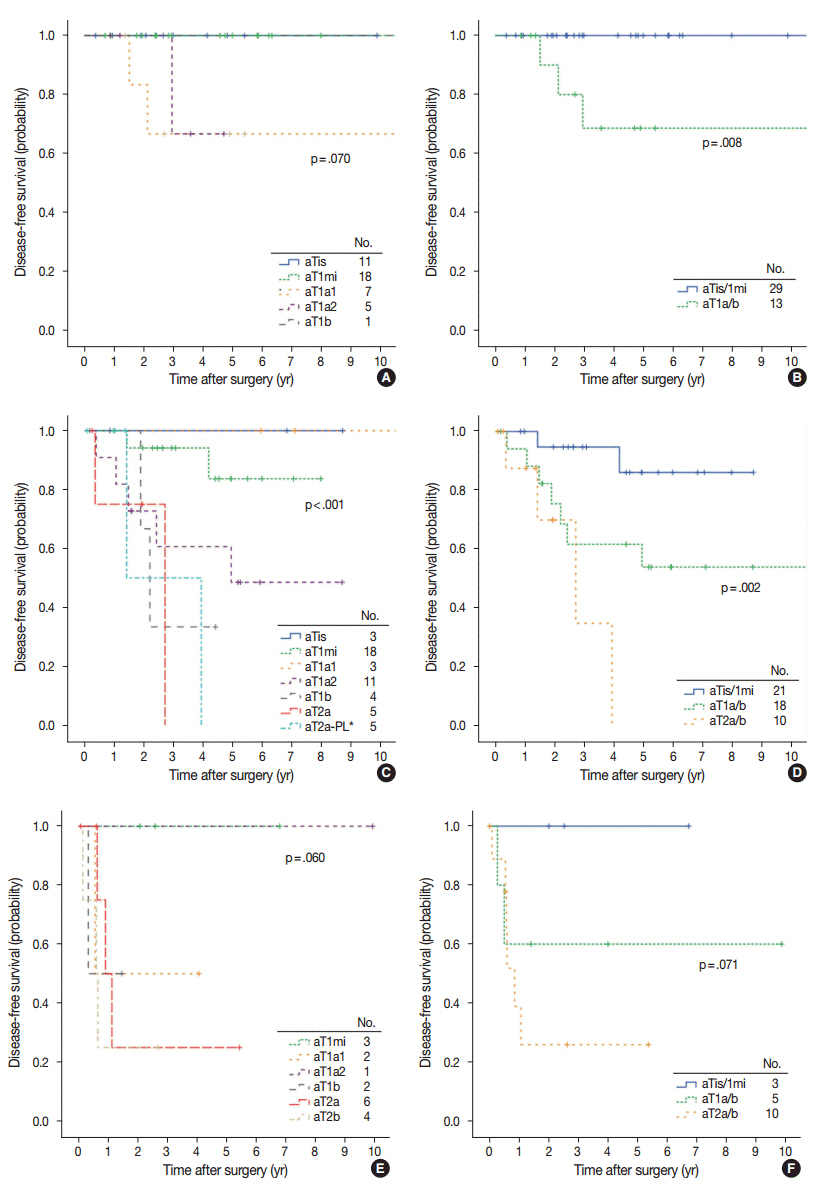
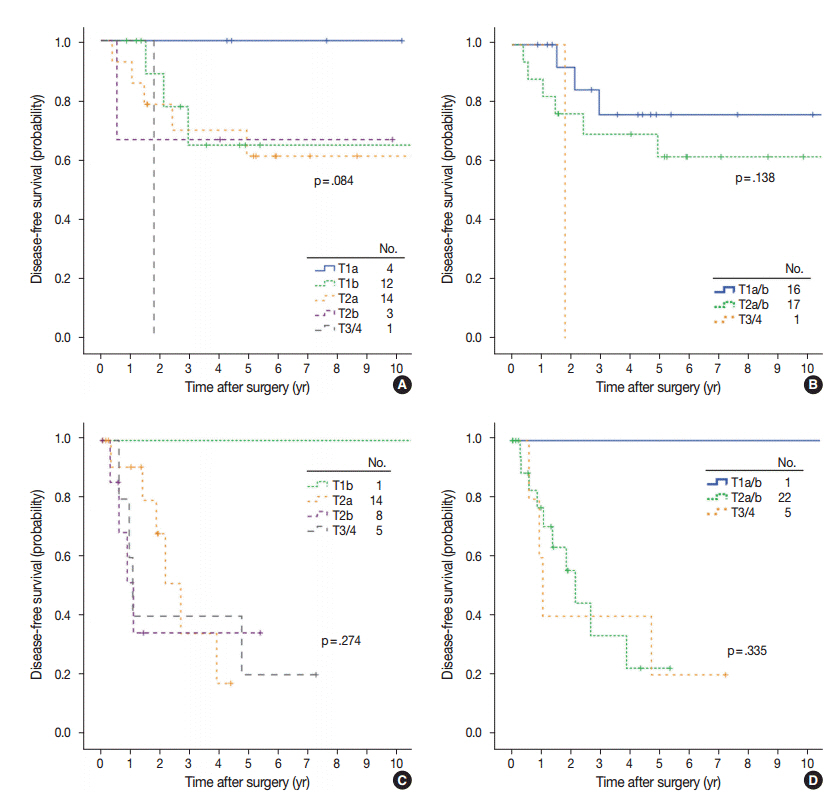
The mean proportion of invasive patterns (patterns 2 and 3) was lowest in small (≤ 3 cm) tumors, and gradually increased in intermediate (> 3 cm and ≤ 7 cm) and large (> 7 cm) tumors (8.4%, 34.3%, and 50.1%, respectively). Adjusted T (aT) stage, as determined by the size of invasive patterns, was positively correlated with adverse histologic and clinical features including older age, male sex, and ever smokers. aTis tumors, which were exclusively composed of lepidic pattern (n = 9), or a mixture of lepidic and pattern 1 (n = 40) without any invasive patterns, showed 100% disease- free survival (DFS). The aT1mi tumors, with minimal (≤ 5 mm) invasive patterns (n = 63), showed a 95.2% 5-year DFS, with recurrences (n = 2) limited to tumors greater than 3 cm in total size (n = 23). Both T and aT stage were significantly associated with DFS; however, survival within the separate T-stage subgroups was stratified according to the aT stage, most notably in the intermediatestage subgroups. In multivariate analysis, the size of invasive patterns (p = .020), pleural invasion (p < .001), and vascular invasion (p = .048) were independent predictors of recurrence, whereas total size failed to achieve statistical significance (p = .121).
CONCLUSIONS
This study provides a rationale for histologic risk stratification in pulmonary MA based on the extent of invasive growth patterns with refined criteria for invasion.
MeSH Terms
Figure
Reference
-
1. Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011; 6:244–85.2. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press;2015. 4th.3. Garfield DH, Cadranel J, West HL. Bronchioloalveolar carcinoma: the case for two diseases. Clin Lung Cancer. 2008; 9:24–9.
Article4. Shim HS, Kenudson M, Zheng Z, et al. Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the Lung. J Thorac Oncol. 2015; 10:1156–62.
Article5. Fernandez-Cuesta L, Plenker D, Osada H, et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014; 4:415–22.6. Nakaoku T, Tsuta K, Ichikawa H, et al. Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin Cancer Res. 2014; 20:3087–93.
Article7. Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: a clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011; 6:1496–504.
Article8. Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011; 24:653–64.
Article9. Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012; 30:1438–46.
Article10. Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013; 8:52–61.
Article11. Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol. 2014; 38:448–60.12. Takahashi Y, Ishii G, Aokage K, Hishida T, Yoshida J, Nagai K. Distinctive histopathological features of lepidic growth predominant node-negative adenocarcinomas 3-5 cm in size. Lung Cancer. 2013; 79:118–24.13. Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol. 2004; 28:198–206.14. Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am J Surg Pathol. 2009; 33:462–9.
Article15. Nishino M, Klepeis VE, Yeap BY, et al. Histologic and cytomorphologic features of ALK-rearranged lung adenocarcinomas. Mod Pathol. 2012; 25:1462–72.16. Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol. 2011; 35:1226–34.17. Fukutomi T, Hayashi Y, Emoto K, Kamiya K, Kohno M, Sakamoto M. Low papillary structure in lepidic growth component of lung adenocarcinoma: a unique histologic hallmark of aggressive behavior. Hum Pathol. 2013; 44:1849–58.
Article18. Compton CC, Byrd DR, Garcia-Aguilar J, Kurtzman SH, Olawaiye A, Washington MK. AJCC cancer staging atlas: a companion to the seventh editions of the AJCC cancer staging manual and handbook. New York: Springer-Verlag;2012.19. Warth A, Muley T, Kossakowski CA, et al. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol. 2015; 39:793–801.
Article20. Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol. 2015; 10:806–14.
Article21. Bellocq A, Antoine M, Flahault A, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998; 152:83–92.22. Wislez M, Massiani MA, Milleron B, et al. Clinical characteristics of pneumonic-type adenocarcinoma of the lung. Chest. 2003; 123:1868–77.23. Sun JM, Lira M, Pandya K, et al. Clinical characteristics associated with ALK rearrangements in never-smokers with pulmonary adenocarcinoma. Lung Cancer. 2014; 83:259–64.24. Ichinokawa H, Ishii G, Nagai K, et al. Distinct clinicopathologic characteristics of lung mucinous adenocarcinoma with KRAS mutation. Hum Pathol. 2013; 44:2636–42.25. Kakegawa S, Shimizu K, Sugano M, et al. Clinicopathological features of lung adenocarcinoma with KRAS mutations. Cancer. 2011; 117:4257–66.26. Sato S, Motoi N, Hiramatsu M, et al. Pulmonary adenocarcinoma in situ: analyses of a large series with reference to smoking, driver mutations, and receptor tyrosine kinase pathway activation. Am J Surg Pathol. 2015; 39:912–21.27. Ichinokawa H, Ishii G, Nagai K, et al. Clinicopathological characteristics of primary lung adenocarcinoma predominantly composed of goblet cells in surgically resected cases. Pathol Int. 2011; 61:423–9.
Article28. Manning JT Jr, Spjut HJ, Tschen JA. Bronchioloalveolar carcinoma: the significance of two histopathologic types. Cancer. 1984; 54:525–34.
Article29. Oka S, Hanagiri T, Uramoto H, et al. Surgical resection for patients with mucinous bronchioloalveolar carcinoma. Asian J Surg. 2010; 33:89–93.
Article30. Suzuki K, Yokose T, Yoshida J, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2000; 69:893–7.
Article31. Borczuk AC. Assessment of invasion in lung adenocarcinoma classification, including adenocarcinoma in situ and minimally invasive adenocarcinoma. Mod Pathol. 2012; 25 Suppl 1:S1–10.32. Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma: an international interobserver study. Mod Pathol. 2012; 25:1574–83.
Article33. Warth A, Stenzinger A, von Brünneck AC, et al. Interobserver variability in the application of the novel IASLC/ATS/ERS classification for pulmonary adenocarcinomas. Eur Respir J. 2012; 40:1221–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Transforming Growth Factor beta1 and Cadherins in Lung Adenocarcinoma
- Solitary Nodular Invasive Mucinous Adenocarcinoma of the Lung: Imaging Diagnosis Using the Morphologic-Metabolic Dissociation Sign
- Clinical Outcome of Surgically Resected Pancreatic Intraductal Papillary Mucinous Neoplasm According to the Marginal Status: A Single Center Experience
- Two cases of mucinous adenocarcinoma of the stomach mistaken as submucosal tumor
- Clinicopathologic Review of 41 Cases of Pancreatic Mucinous Cystic Neoplasms