Clin Exp Vaccine Res.
2017 Jan;6(1):45-49. 10.7774/cevr.2017.6.1.45.
Trivalent M-related protein as a component of next generation group A streptococcal vaccines
- Affiliations
-
- 1Department of Medicine, Immunology and Biochemistry, The University of Tennessee Health Science Center, Memphis, TN, USA. jbdale@uthsc.edu
- 2Department of Medicine, Veterans Affairs Medical Center, Memphis, TN, USA.
- 3Department of Microbiology, Immunology and Biochemistry, The University of Tennessee Health Science Center, Memphis, TN, USA.
- KMID: 2366891
- DOI: http://doi.org/10.7774/cevr.2017.6.1.45
Abstract
- PURPOSE
There is a need to broaden protective coverage of M protein-based vaccines against group A streptococci (GAS) because coverage of the current 30-valent M protein vaccine does not extend to all emm types. An additional GAS antigen and virulence factor that could potentially extend vaccine coverage is M-related protein (Mrp). Previous work indicated that there are three structurally related families of Mrp (MrpI, MrpII, and MrpIII) and peptides of all three elicited bactericidal antibodies against multiple emm types. The purpose of this study was to determine if a recombinant form containing Mrp from the three families would evoke bactericidal antiserum and to determine if this antiserum could enhance the effectiveness of antisera to the 30-valent M protein vaccine.
MATERIALS AND METHODS
A trivalent recombinant Mrp (trMrp) protein containing N-terminal fragments from the three families (trMrp) was constructed, purified and used to immunize rabbits. Anti-trMrp sera contained high titers of antibodies against the trMrp immunogen and recombinant forms representing MrpI, MrpII, and MrpIII.
RESULTS
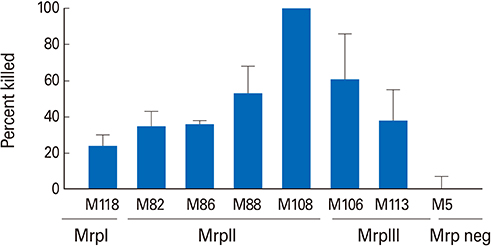
The antisera opsonized emm types of GAS representing each Mrp family and also opsonized emm types not covered by the 30-valent M protein-based vaccine. Importantly, a combination of trMrp and 30-valent M protein antiserum resulted in higher levels of opsonization of GAS than either antiserum alone.
CONCLUSION
These findings suggest that trMrp may be an effective addition to future constructs of GAS vaccines.
Keyword
MeSH Terms
Figure
Reference
-
1. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005; 5:685–694.
Article2. Dale JB, Batzloff MR, Cleary PP, Courtney HS, Good MF, Grandi G, et al. Current approaches to group A streptococcal vaccine development. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations [Internet]. Oklahoma City: University of Oklahoma Health Sciences Center;cited 2016 Dec 1. Available from: https://www.ncbi.nlm.nih.gov/books/NBK333413/.3. Dale JB, Penfound TA, Tamboura B, et al. Potential coverage of a multivalent M protein-based group A streptococcal vaccine. Vaccine. 2013; 31:1576–1581.
Article4. Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine. 2011; 29:8175–8178.
Article5. Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009; 9:611–616.
Article6. Courtney HS, Hasty DL, Dale JB. Anti-phagocytic mechanisms of Streptococcus pyogenes: binding of fibrinogen to M-related protein. Mol Microbiol. 2006; 59:936–947.
Article7. Courtney HS, Li Y. Non-immune binding of human IgG to M-related proteins confers resistance to phagocytosis of group A streptococci in blood. PLoS One. 2013; 8:e78719.
Article8. Podbielski A, Schnitzler N, Beyhs P, Boyle MD. M-related protein (Mrp) contributes to group A streptococcal resistance to phagocytosis by human granulocytes. Mol Microbiol. 1996; 19:429–441.
Article9. Li Y, Courtney HS. Promotion of phagocytosis of Streptococcus pyogenes in human blood by a fibrinogen-binding peptide. Microbes Infect. 2011; 13:413–418.
Article10. Dale JB, Niedermeyer SE, Agbaosi T, et al. Protective immunogenicity of group A streptococcal M-related proteins. Clin Vaccine Immunol. 2015; 22:344–350.
Article11. Niedermeyer SE, Penfound TA, Hohn C, et al. Group A streptococcus expresses a trio of surface proteins containing protective epitopes. Clin Vaccine Immunol. 2014; 21:1421–1425.
Article12. Lancefield RC. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J Exp Med. 1957; 106:525–544.
Article13. McNeil SA, Halperin SA, Langley JM, et al. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005; 41:1114–1122.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Accelerating the development of a group A Streptococcus vaccine: an urgent public health need
- Parenteral, non-live rotavirus vaccine: recent history and future perspective
- Influenza Vaccines: Unmet Needs and Recent Developments
- Clinical study of group B streptococcal infection in infants less than two months of age
- Development and trial of vaccines against Brucella