J Lipid Atheroscler.
2016 Dec;5(2):107-113. 10.12997/jla.2016.5.2.107.
Novel Molecular Basis for Vascular Health Regulated by Vasohibin-1
- Affiliations
-
- 1Department of Vascular Biology, Institute of Development, Aging and Cancer, Tohoku University, Japan. yasufumi.sato.b3@tohoku.ac.jp
- KMID: 2366135
- DOI: http://doi.org/10.12997/jla.2016.5.2.107
Abstract
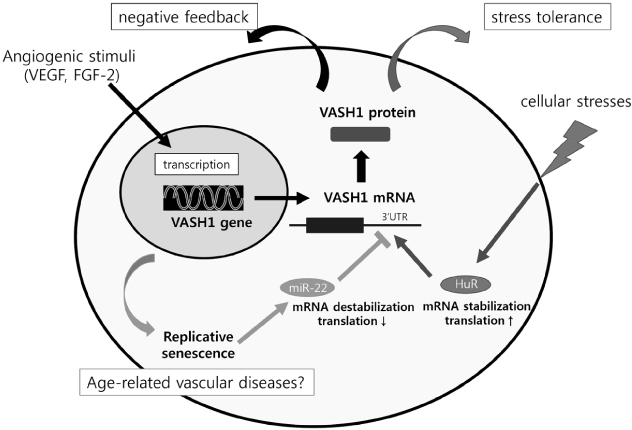
- The endothelium covers the entire luminal surface of blood vessels, organizes the interface between the blood and underlying tissues, and controls vascular tone, blood clotting, transport of various substances across the vascular wall, adhesion and transmigration of leukocytes, and so forth. The structural and functional integrity of endothelium is essential for the maintenance of vascular health. In light of its important role, the endothelium should have a self-defense system such as vasohibin-1 (VASH1), a protein preferentially expressed in endothelial cells (ECs). Unique features of VASH1 are its anti-angiogenic activity and ability to promote stress tolerance of ECs. This mini review summarizes the current understanding of VASH1, especially the posttranscriptional regulation of its synthesis in response to cellular stresses and aging.
MeSH Terms
Figure
Reference
-
1. Hazzard WR. Atherosclerosis and aging: a scenario in flux. Am J Cardiol. 1989; 63:20H–24H.
Article2. Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016; 594:2061–2073.
Article3. Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab. 2012; 302:E481–E495.
Article4. Lin MI, Sessa WC. Vascular endothelial growth factor signaling to endothelial nitric oxide synthase: more than a FLeeTing moment. Circ Res. 2006; 99:666–668.
Article5. Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007; 130:691–703.
Article6. Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M, et al. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest. 2004; 114:898–907.
Article7. Miyashita H, Watanabe T, Hayashi H, Suzuki Y, Nakamura T, Ito S, et al. Angiogenesis inhibitor vasohibin-1 enhances stress resistance of endothelial cells via induction of SOD2 and SIRT1. PLoS One. 2012; 7:e46459.
Article8. Abe M, Sato Y. cDNA microarray analysis of the gene expression profile of VEGF-activated human umbilical vein endothelial cells. Angiogenesis. 2001; 4:289–298.9. Shibuya T, Watanabe K, Yamashita H, Shimizu K, Miyashita H, Abe M, et al. Isolation and characterization of vasohibin-2 as a homologue of VEGF-inducible endothelium-derived angiogenesis inhibitor vasohibin. Arterioscler Thromb Vasc Biol. 2006; 26:1051–1057.
Article10. Sato Y. The vasohibin family: Novel regulators of angiogenesis. Vascul Pharmacol. 2012; 56:262–266.
Article11. Jennings BJ, Ozanne SE, Hales CN. Nutrition, oxidative damage, telomere shortening, and cellular senescence: individual or connected agents of aging? Mol Genet Metab. 2000; 71:32–42.
Article12. Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci. 2013; 34:313–319.
Article13. Kimura H, Miyashita H, Suzuki Y, Kobayashi M, Watanabe K, Sonoda H, et al. Distinctive localization and opposed roles of vasohibin-1 and vasohibin-2 in the regulation of angiogenesis. Blood. 2009; 113:4810–4818.
Article14. Ito S, Miyashita H, Suzuki Y, Kobayashi M, Satomi S, Sato Y. Enhanced cancer metastasis in mice deficient in vasohibin-1 gene. PLoS One. 2013; 8:e73931.
Article15. Bir SC, Kolluru GK, Fang K, Kevil CG. Redox balance dynamically regulates vascular growth and remodeling. Semin Cell Dev Biol. 2012; 23:745–757.
Article16. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011; 15:1583–1606.
Article17. Suzuki K, Tatsumi H, Satoh S, Senda T, Nakata T, Fujii J, et al. Manganese-superoxide dismutase in endothelial cells: localization and mechanism of induction. Am J Physiol. 1993; 265:H1173–H1178.
Article18. El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. 2013; 65:380–401.
Article19. Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005; 6:298–305.
Article20. Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005; 120:473–482.21. Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014; 25:138–145.
Article22. Gorenne I, Kumar S, Gray K, Figg N, Yu H, Mercer J, et al. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. 2013; 127:386–396.
Article23. Orimo M, Minamino T, Miyauchi H, Tateno K, Okada S, Moriya J, et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009; 29:889–894.
Article24. Takemura A, Iijima K, Ota H, Son BK, Ito Y, Ogawa S, et al. Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011; 31:2054–2062.
Article25. Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008; 80:191–199.
Article26. Shimizu K, Watanabe K, Yamashita H, Abe M, Yoshimatsu H, Ohta H, et al. Gene regulation of a novel angiogenesis inhibitor, vasohibin, in endothelial cells. Biochem Biophys Res Commun. 2005; 327:700–706.
Article27. Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008; 65:3168–3181.
Article28. Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle. 2007; 6:1288–1292.
Article29. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009; 136:215–233.
Article30. Breving K, Esquela-Kerscher A. The complexities of microRNA regulation: mirandering around the rules. Int J Biochem Cell Biol. 2010; 42:1316–1329.
Article31. Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012; 111:245–259.32. Lee S, Choi E, Cha MJ, Park AJ, Yoon C, Hwang KC. Impact of miRNAs on cardiovascular aging. J Geriatr Cardiol. 2015; 12:569–574.33. Takeda E, Suzuki Y, Sato Y. Age-associated downregulation of vasohibin-1 in vascular endothelial cells. Aging Cell. 2016; 15:885–892.
Article34. Hinamoto N, Maeshima Y, Yamasaki H, Nasu T, Saito D, Watatani H, et al. Exacerbation of diabetic renal alterations in mice lacking vasohibin-1. PLoS One. 2014; 9:e107934.
Article35. Nasu T, Maeshima Y, Kinomura M, Hirokoshi-Kawahara K, Tanabe K, Sugiyama H, et al. Vasohibin-1, a negative feedback regulator of angiogenesis, ameliorates renal alterations in a mouse model of diabetic nephropathy. Diabetes. 2009; 58:2365–2375.
Article36. Saito D, Maeshima Y, Nasu T, Yamasaki H, Tanabe K, Sugiyama H, et al. Amelioration of renal alterations in obese type 2 diabetic mice by vasohibin-1, a negative feedback regulator of angiogenesis. Am J Physiol Renal Physiol. 2011; 300:F873–F886.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiovascular Molecular Imaging with Contrast Ultrasound: Principles and Applications
- Molecular basis and diagnosis of thalassemia
- Studies on the Mechanism of Hypoxic Increase of VEGF Expression in the Hep3B Human Hepatoma Cells
- Intimal Hyperplasia in Vascular Grafts: Surgery-induced Arteriosclerosis
- Regulatory Effects of O-GlcNAcylation in Vascular Smooth Muscle Cells on Diabetic Vasculopathy