J Korean Neurosurg Soc.
2017 Jan;60(1):40-46. 10.3340/jkns.2015.0911.002.
Role of Expression of Inflammatory Mediators in Primary and Recurrent Lumbar Disc Herniation
- Affiliations
-
- 1Department of Neurosurgery, Abant Izzet Baysal University Medical School, Izzet Baysal Training and Research Hospital, Bolu, Turkey. dagistanyasar@hotmail.com
- 2Department of Pathology, Abant Izzet Baysal University Medical School, Izzet Baysal Training and Research Hospital, Bolu, Turkey.
- 3Department of Radiology, Abant Izzet Baysal University Medical School, Izzet Baysal Training and Research Hospital, Bolu, Turkey.
- KMID: 2365585
- DOI: http://doi.org/10.3340/jkns.2015.0911.002
Abstract
OBJECTIVE
To assess role of some inflammatory mediators in patients with primary and recurrent lumbar disc herniation. Expression of IL-6, transforming growth factor (TGF)-1, insulin-like growth factor (IGF)-1, and Bcl-2-associated X protein (BAX) have been shown to be more intense in the primary group than the recurrent goup, but this mediators may be important aspects prognostic.
METHODS
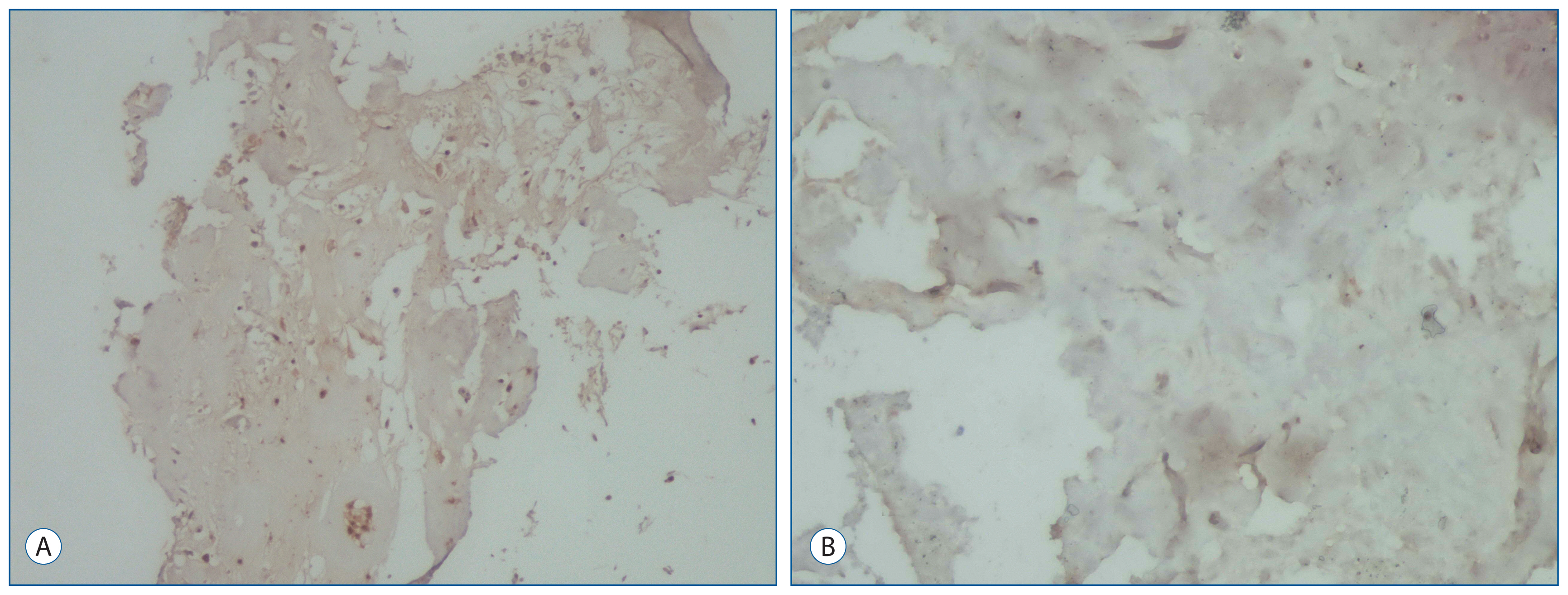
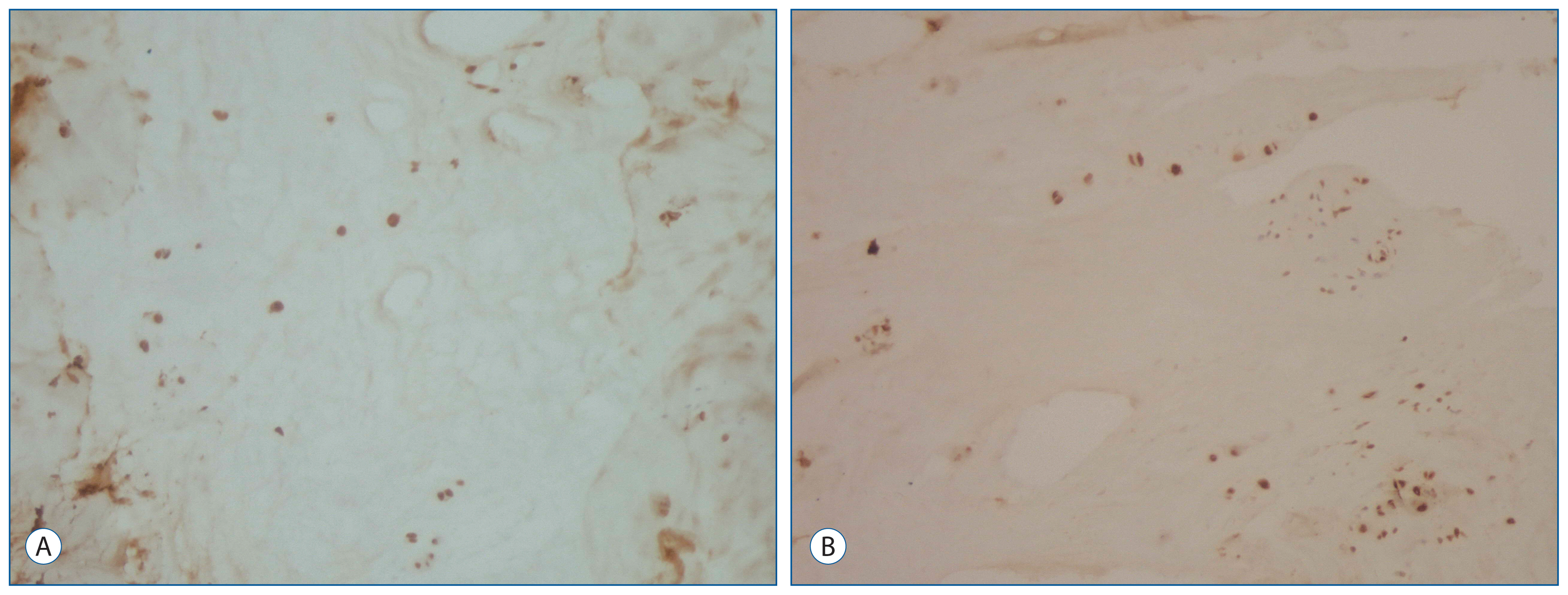
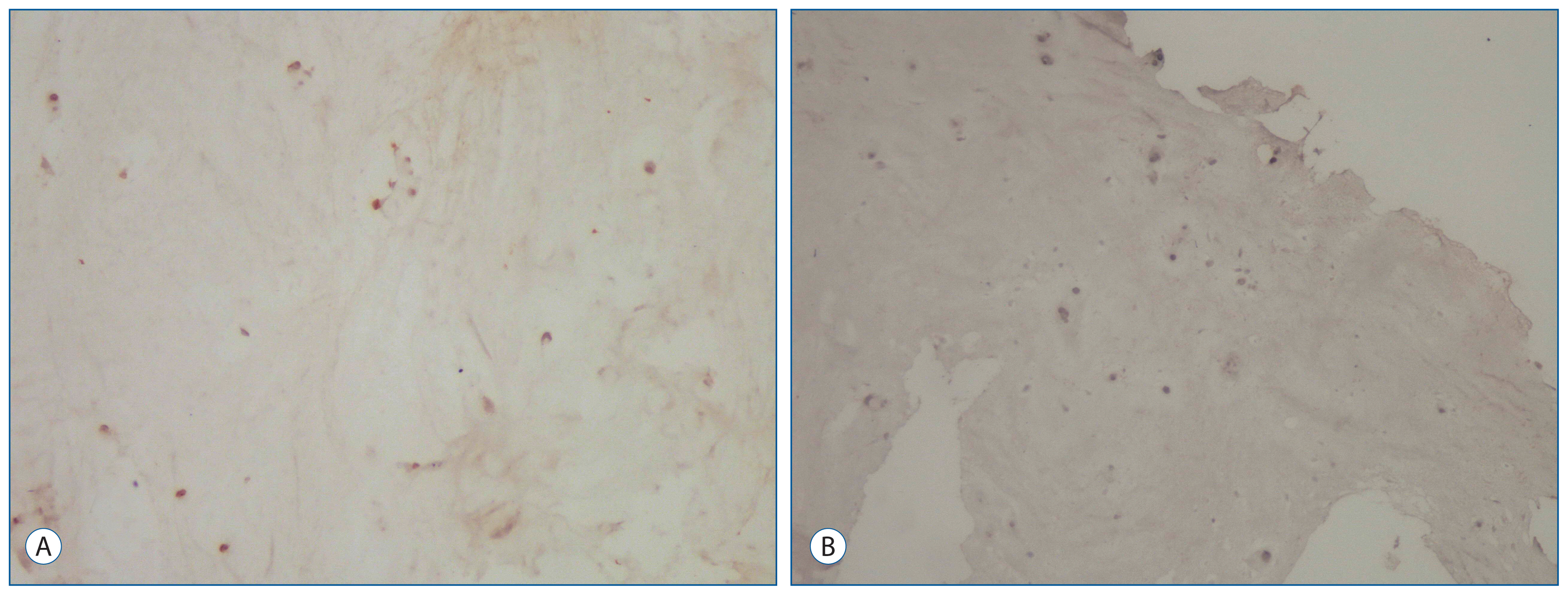
19 patients underwent primary and revision operations between June 1, 2009 and June 1, 2014, and they were included in this study. The 19 patients' intervertebral disc specimens obtained from the primary procedures and reoperations were evaluated. Expression of IL-6, TGF-1, IGF-1, and BAX were examined immunohistochemically in the 38 biopsy tissues obtained from the primary and recurrent herniated intervertebral discs during the operation.
RESULTS
For IL-6 expression in the intervertebral disc specimens, there was no difference between the groups. The immunohistochemical study showed that the intervertebral disc specimens in the primary group were stained intensely by TGF-1 compared with the recurrent group. Expression of IGF-1 in the primary group was found moderate. In contrast, in the recurrent group of patients was mild expression of IGF-1. The primary group intervertebral disc specimens were stained moderately by BAX compared with the recurrent group.
CONCLUSION
The results of our prognostic evaluation of patients in the recurrent group who were operated due to disc herniation suggest that mediators may be important parameters.
MeSH Terms
Figure
Reference
-
References
1. Aizawa T, Ozawa H, Kusakabe T, Nakamura T, Sekiguchi A, Takahashi A, et al. Reoperation for recurrent lumbar disc herniation: a study over a 20-year period in a Japanese population. J Orthop Sci. 17:107–113. 2012.
Article2. Ambrossi GLG, McGirt MJ, Sciubba DM, Witham TF, Wolinsky JP, Gokaslan ZL, et al. Recurrent lumbar disc herniation after single-level lumbar discectomy: incidence and health care cost analysis. Neurosurgery. 65:574–578. discussion 578. 2009.3. Babar S, Saifuddin A. MRI of the post-discectomy lumbar spine. Clin Radiol. 57:969–981. 2002.
Article4. Burke JG, Watson RWG, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 84:196–201. 2002.
Article5. Cheng JW, Wang HW, Zheng WJ, Li CQ, Wang J, Zhang ZF, et al. Reoperation after lumbar disc surgery in two hundred and seven patients. Int Orthop. 37:1511–1517. 2013.
Article6. Fandino J, Botana C, Viladrich A, Gomezbueno J. Reoperation after lumbar disc surgery - results in 130 cases. Acta Neurochir (Wien). 122:102–104. 1993.7. Gibson JNA, Waddell G. Surgical interventions for lumbar disc prolapse - updated cochrane review. Spine. 32:1735–1747. 2007.8. Gruber HE, Fisher EC Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN Jr. Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta 1. Exp Cell Res. 235:13–21. 1997.
Article9. Heyde CE, Tschoeke SK, Hellmuth M, Hostmann A, Ertel W, Oberholzer A. Trauma induces apoptosis in human thoracolumbar intervertebral discs. BMC Clin Pathol. 6:5. 2006.
Article10. Jennische E, Skottner A, Hansson HA. Dynamic changes in insulin-like growth factor I immunoreactivity correlate to repair events in rat ear after freeze-thaw injury. Exp Mol Pathol. 47:193–201. 1987.
Article11. Kara B, Tulum Z, Acar U. Functional results and the risk factors of reoperations after lumbar disc surgery. Eur Spine J. 14:43–48. 2005.
Article12. Kiechle FL, Zhang XB. Apoptosis: biochemical aspects and clinical implications. Clin Chim Acta. 326:27–45. 2002.
Article13. Kinkel MD, Horton WE Jr. Coordinate down-regulation of cartilage matrix gene expression in Bcl-2 deficient chondrocytes is associated with decreased SOX9 expression and decreased mRNA stability. J Cell Biochem. 88:941–953. 2003.
Article14. Korsmeyer SJ, Wei MC, Saito M, Weller S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 7:1166–1173. 2000.
Article15. Laiho M, Keski-Oja J. Growth factors in the regulation of pericellular proteolysis: a review. Cancer Res. 49:2533–2553. 1989.16. Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 9:R77. 2007.17. Matsumoto M, Watanabe K, Hosogane N, Tsuji T, Ishii K, Nakamura M, et al. Recurrence of Lumbar Disc Herniation after Microendoscopic Discectomy. J Neurol Surg A Cent Eur Neurosurg. 74:222–227. 2013.
Article18. Morgan-Hough CV, Jones PW, Eisenstein SM. Primary and revision lumbar discectomy - a 16-year review from one centre. J Bone Joint Surg Br. 85:871–874. 2003.19. Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 15:879–892. 2001.
Article20. Ohtori S, Suzuki M, Koshi T, Takaso M, Yamashita M, Inoue G, et al. Proinflammatory cytokines in the cerebrospinal fluid of patients with lumbar radiculopathy. Eur Spine J. 20:942–946. 2011.
Article21. Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, et al. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 14:690–699. 1996.
Article22. Pattison ST, Melrose J, Ghosh P, Taylor TK. Regulation of gelatinase-A (MMP-2) production by ovine intervertebral disc nucleus pulposus cells grown in alginate bead culture by transforming growth factor-beta(1)and insulin like growth factor-I. Cell Biol Int. 25:679–689. 2001.
Article23. Phillip M, Maor G, Assa S, Silbergeld A, Segev Y. Testosterone stimulates growth of tibial epiphyseal growth plate and insulin-like growth factor-1 receptor abundance in hypophysectomized and castrated rats. Endocrine. 16:1–6. 2001.
Article24. Postlethwaite AE, Keskioja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 165:251–256. 1987.
Article25. Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 83:4167–4171. 1986.
Article26. Schwander JC, Hauri C, Zapf J, Froesch ER. Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology. 113:297–305. 1983.
Article27. Specchia N, Pagnotta A, Toesca A, Greco F. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur Spine J. 11:145–151. 2002.
Article28. Suk KS, Lee HM, Moon SH, Kim NH. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976). 26:672–676. 2001.29. Takada T, Nishida K, Doita M, Miyamoto H, Kurosaka M. Interleukin-6 production is upregulated by interaction between disc tissue and macrophages. Spine (Phila Pa 1976). 29:1089–1092. discussion 1093. 2004.
Article30. Thompson JP, Oegema TR Jr, Bradford DS. Stimulation of mature canine intervertebral-disk by growth-factors. Spine (Phila Pa 1976). 16:253–260. 1991.31. Uceyler N, Kafke W, Riediger N, He L, Necula G, Toyka KV, et al. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology. 74:1806–1813. 2010.
Article32. von Ahsen O, Renken C, Perkins G, Kluck RM, Bossy-Wetzel E, Newmeyer DD. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J Cell Biol. 150:1027–1036. 2000.
Article33. Wang HL, Ahrens C, Rief W, Gantz S, Schiltenwolf M, Richter W. Influence of depression symptoms on serum tumor necrosis factor-alpha of patients with chronic low back pain. Arthritis Res Ther. 12:R186. 2010.34. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 292:727–730. 2001.
Article35. Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Phila Pa 1976). 30:44–53. 2005.
Article36. Wera GD, Marcus RE, Ghanayem AJ, Bohlman HH. Failure within one year following subtotal lumbar discectomy. J Bone Joint Surg Am. 90:10–15. 2008.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid Repeated Recurrent Lumbar Disc Herniation after Microscopic Discectomies
- Clinical Analysis of Recurrent Lumbar Disc Herniation
- Different Expression of Extracellular Matrix Genes: Primary vs. Recurrent Disc Herniation
- Transforaminal Endoscopic Lumbar Discectomy With Bone Drill-Assisted Foraminoplasty in Recurrent Disc Herniation
- Clinical Outcome and Influencing Factor for Repeat Lumbar Discectomy for Ipsilateral Recurrent Lumbar Disc Herniation