J Clin Neurol.
2016 Jan;12(1):101-106. 10.3988/jcn.2016.12.1.101.
Real-Time Quaking-Induced Conversion Analysis for the Diagnosis of Sporadic Creutzfeldt-Jakob Disease in Korea
- Affiliations
-
- 1Korea CJD Diagnostic Center, Hallym University, Anyang, Korea. yskim@hallym.ac.kr
- 2Laboratory of Transmissible Spongiform Encephalopathy, Ilsong Institute of Life Science, Hallym University, Anyang, Korea.
- 3Department of Pathology, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea.
- 4Korea CJD Autopsy Center, Hallym University Sacred Heart Hospital, Anyang, Korea.
- 5Department of Biomedical Gerontology, Graduate School of Hallym University, Chuncheon, Korea. ekchoi@hallym.ac.kr
- 6Laboratory of Cellular Aging and Neurodegeneration, Ilsong Institute of Life Science, Hallym University, Anyang, Korea.
- KMID: 2364926
- DOI: http://doi.org/10.3988/jcn.2016.12.1.101
Abstract
- BACKGROUND AND PURPOSE
The level of 14-3-3 protein in the cerebrospinal fluid (CSF) is increased in Creutzfeldt-Jakob disease (CJD) patients, which has led to it being used as a clinical biomarker for the ante-mortem diagnosis of human prion diseases. However, the specificity of the 14-3-3 protein is less reliable for CJD diagnosis. Newly developed assays including real-time quaking-induced conversion (RT-QuIC) have made it possible to detect the PrPSc-like abnormal prion isoform with a high sensitivity in animal and human specimens that might contain a minute amount of PrP(Sc) due to in vitro prion replication.
METHODS
This study applied a highly sensitive RT-QuIC assay using recombinant human PrP to detect PrP(Sc) in the CSF of 81 patients with sporadic CJD (sCJD) in Korea.
RESULTS
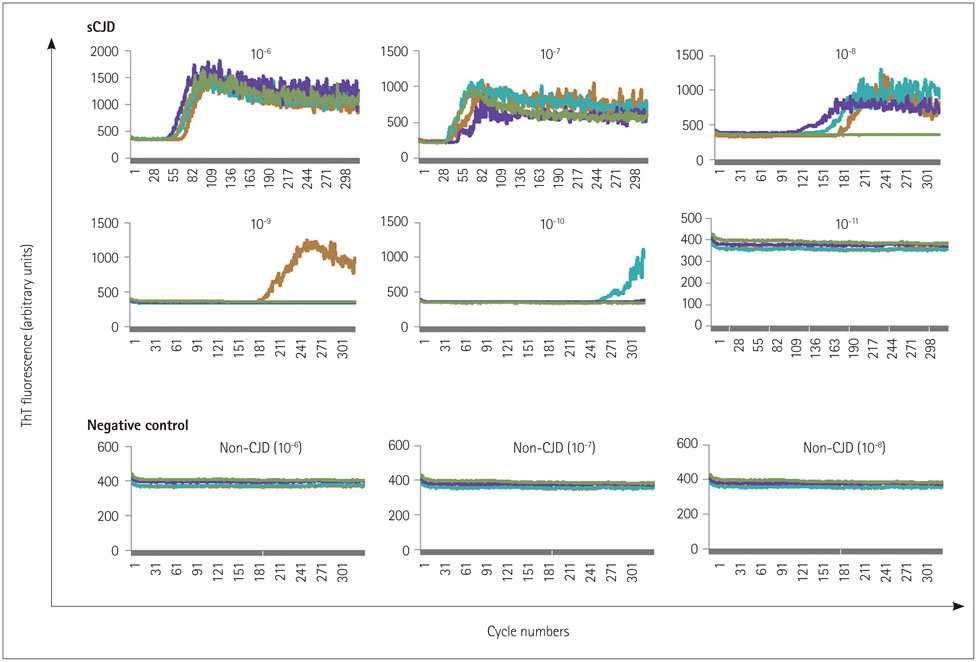
RT-QuIC analysis of the CSF samples based on the expression levels of 14-3-3 and total tau proteins revealed positivity in 62 of 81 sCJD patients (sensitivity of 76.5%) but no positive results in the 100 non-CJD patients.
CONCLUSIONS
The sensitivity of the RT-QuIC in this study was similar to that in some previous reports, and the specificity of RT-QuIC was higher than that of 14-3-3 in CSF, suggesting that RT-QuIC analysis can complement the weakness of the specificity of 14-3-3 for the diagnosis of sCJD. These results indicate that RT-QuIC might be very useful for the rapid and specific diagnosis of sCJD and provide a practical novel method for the ante-mortem diagnosis of human prion diseases.
MeSH Terms
Figure
Reference
-
1. Kübler E, Oesch B, Raeber AJ. Diagnosis of prion diseases. Br Med Bull. 2003; 66:267–279.
Article2. Yang TI, Jung DS, Ahn BY, Jeong BH, Cho HJ, Kim YS, et al. Familial Creutzfeldt-Jakob disease with V180I mutation. J Korean Med Sci. 2010; 25:1097–1100.
Article3. Tschampa HJ, Zerr I, Urbach H. Radiological assessment of Creutzfeldt-Jakob disease. Eur Radiol. 2007; 17:1200–1211.
Article4. Shimada T, Fournier AE, Yamagata K. Neuroprotective function of 14-3-3 proteins in neurodegeneration. Biomed Res Int. 2013; 2013:564534.
Article5. Stoeck K, Sanchez-Juan P, Gawinecka J, Green A, Ladogana A, Pocchiari M, et al. Cerebrospinal fluid biomarker supported diagnosis of Creutzfeldt-Jakob disease and rapid dementias: a longitudinal multicentre study over 10 years. Brain. 2012; 135(Pt 10):3051–3061.
Article6. Aguzzi A, Heikenwalder M, Miele G. Progress and problems in the biology, diagnostics, and therapeutics of prion diseases. J Clin Invest. 2004; 114:153–160.
Article7. Cramm M, Schmitz M, Karch A, Zafar S, Varges D, Mitrova E, et al. Characteristic CSF prion seeding efficiency in humans with prion diseases. Mol Neurobiol. 2015; 51:396–405.
Article8. Orrù CD, Wilham JM, Vascellari S, Hughson AG, Caughey B. New generation QuIC assays for prion seeding activity. Prion. 2012; 6:147–152.
Article9. Peden AH, McGuire LI, Appleford NE, Mallinson G, Wilham JM, Orrú CD, et al. Sensitive and specific detection of sporadic Creutzfeldt-Jakob disease brain prion protein using real-time quaking-induced conversion. J Gen Virol. 2012; 93(Pt 2):438–449.
Article10. LeVine H 3rd. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999; 309:274–284.11. Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 2011; 17:175–178.
Article12. Atarashi R, Sano K, Satoh K, Nishida N. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion. 2011; 5:150–153.13. Jeong BH, Nam JH, Lee YJ, Lee KH, Jang MK, Carp RI, et al. Polymorphisms of the prion protein gene (PRNP) in a Korean population. J Hum Genet. 2004; 49:319–324.
Article14. Skillbäck T, Rosén C, Asztely F, Mattsson N, Blennow K, Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol. 2014; 71:476–483.
Article15. Sano K, Satoh K, Atarashi R, Takashima H, Iwasaki Y, Yoshida M, et al. Early detection of abnormal prion protein in genetic human prion diseases now possible using real-time QUIC assay. PLoS One. 2013; 8:e54915.
Article16. Orrú CD, Bongianni M, Tonoli G, Ferrari S, Hughson AG, Groveman BR, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014; 371:519–529.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of Creutzfeldt-Jakob disease
- A Case of Probable Creutzfeldt-Jakob Disease with Coexistence of the Features of Hashimoto Encephalopathy
- New Variant Creutzfeldt-Jakob Disease
- Familial Creutzfeldt-Jakob Disease with V180I Mutation Presented with Broca's Aphasia
- Anesthetic management in patients suspected of Creutzfeldt-Jakob disease -A case report-