J Korean Med Sci.
2016 Feb;31(Suppl 1):S10-S23. 10.3346/jkms.2016.31.S1.S10.
Is the Linear No-Threshold Dose-Response Paradigm Still Necessary for the Assessment of Health Effects of Low Dose Radiation?
- Affiliations
-
- 1Laboratory of Radiation Exposure & Therapeutics, National Radiation Emergency Medical Center, Korea Institute of Radiological & Medical Sciences, Seoul, Korea. ywjin@kirams.re.kr
- KMID: 2363376
- DOI: http://doi.org/10.3346/jkms.2016.31.S1.S10
Abstract
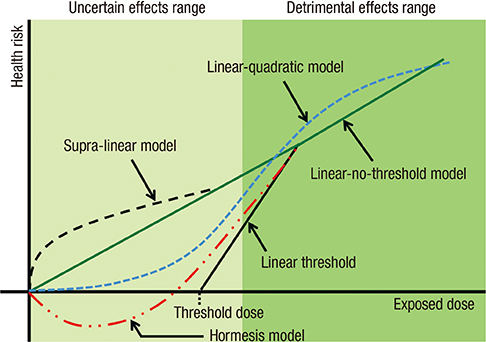
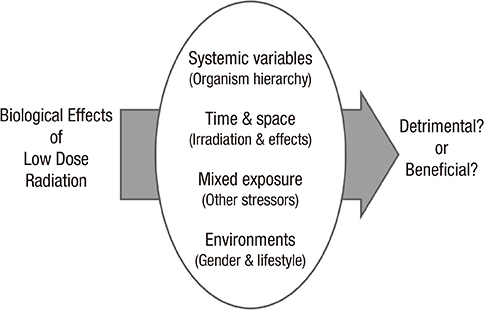
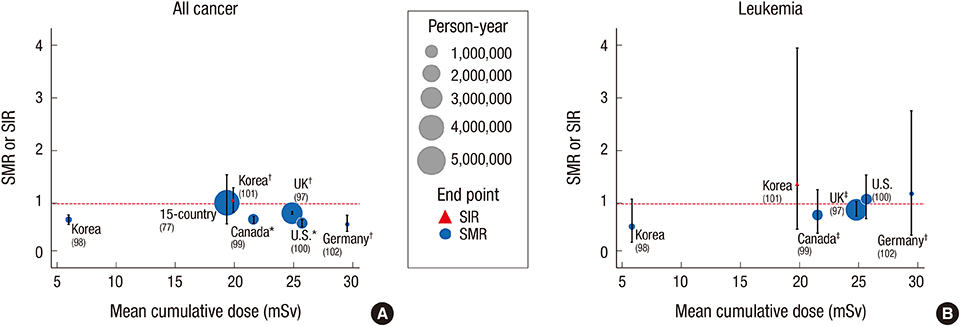
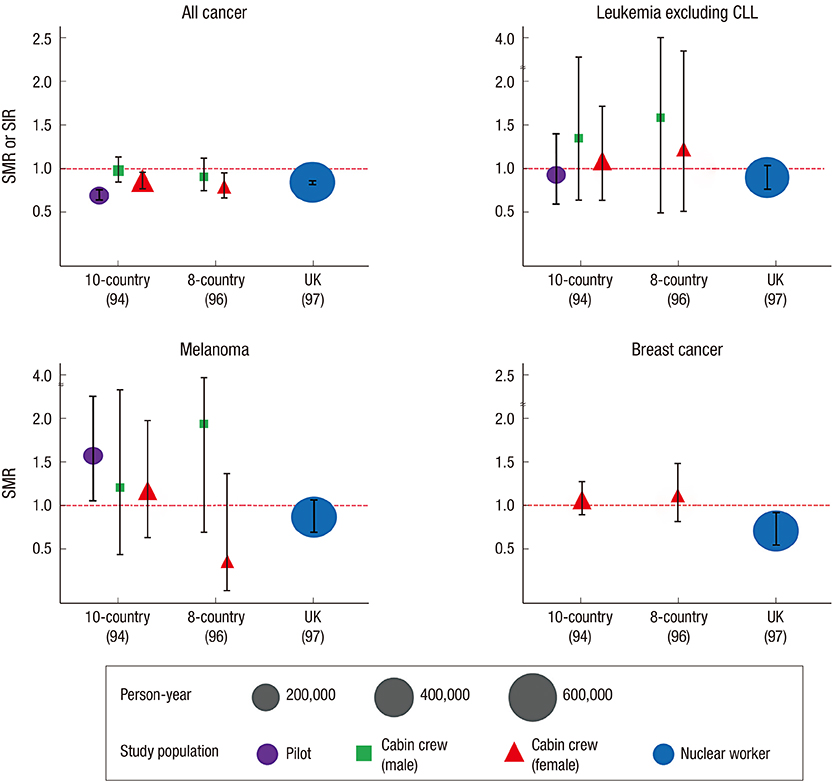
- Inevitable human exposure to ionizing radiation from man-made sources has been increased with the proceeding of human civilization and consequently public concerns focus on the possible risk to human health. Moreover, Fukushima nuclear power plant accidents after the 2011 East-Japan earthquake and tsunami has brought the great fear and anxiety for the exposure of radiation at low levels, even much lower levels similar to natural background. Health effects of low dose radiation less than 100 mSv have been debated whether they are beneficial or detrimental because sample sizes were not large enough to allow epidemiological detection of excess effects and there was lack of consistency among the available experimental data. We have reviewed an extensive literature on the low dose radiation effects in both radiation biology and epidemiology, and highlighted some of the controversies therein. This article could provide a reasonable view of utilizing radiation for human life and responding to the public questions about radiation risk. In addition, it suggests the necessity of integrated studies of radiobiology and epidemiology at the national level in order to collect more systematic and profound information about health effects of low dose radiation.
MeSH Terms
Figure
Cited by 1 articles
-
Prospects on the increase of radiological examinations in Korea
Joon-Il Choi
J Korean Med Assoc. 2020;63(3):136-139. doi: 10.5124/jkma.2020.63.3.136.
Reference
-
1. Lederman M. The early history of radiotherapy: 1895-1939. Int J Radiat Oncol Biol Phys. 1981; 7:639–648.2. Mettler FA. Medical effects and risks of exposure to ionising radiation. J Radiol Prot. 2012; 32:N9–N13.3. Duffin J, Hayter CR. Baring the sole. The rise and fall of the shoe-fitting fluoroscope. Isis. 2000; 91:260–282.4. Preston RJ. Radiation biology: concepts for radiation protection. Health Phys. 2005; 88:545–556.5. Calabrese EJ. Muller’s Nobel Prize Lecture: when ideology prevailed over science. Toxicol Sci. 2012; 126:1–4.6. Calabrese EJ. Key studies used to support cancer risk assessment questioned. Environ Mol Mutagen. 2011; 52:595–606.7. Dauer LT, Brooks AL, Hoel DG, Morgan WF, Stram D, Tran P. Review and evaluation of updated research on the health effects associated with low-dose ionising radiation. Radiat Prot Dosimetry. 2010; 140:103–136.8. Calabrese EJ, O’Connor MK. Estimating risk of low radiation doses - a critical review of the BEIR VII report and its use of the linear no-threshold (LNT) hypothesis. Radiat Res. 2014; 182:463–474.9. National Research Council (US) Committee to Assess Health Risks from Exposure to Low Level of Ionizing Radiation. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, D.C.: The National Academies Press;2006.10. ICRP. Low-dose extrapolation of radiation-related cancer risk. ICRP Publication 99. Ann ICRP. 2005; 35:1–141.11. Tubiana M, Aurengo A, Averbeck D, Bonin A, Le Guen B, Masse R, Monier R, Valleron AJ, de Vathaire F. Dose-effect relationships and estimation of the carcinogenic effect of low doses of ionizing radiation. Paris: Académie des Sciences - Académie National de Médicine;2005.12. United Nations Scientific Committee on the Effects of Atomic Radiation. Biological mechanisms of radiation actions at low doses: a white paper to guide the Scientific Committee's future programme of work. New York, NY: United Nations;2012.13. Goodhead DT. Understanding and characterisation of the risks to human health from exposure to low levels of radiation. Radiat Prot Dosimetry. 2009; 137:109–117.14. Kadhim M, Salomaa S, Wright E, Hildebrandt G, Belyakov OV, Prise KM, Little MP. Non-targeted effects of ionising radiation--implications for low dose risk. Mutat Res. 2013; 752:84–98.15. ICRP. Statement on Tissue Reactions and Early and Late Effects of Radiation in Normal Tissues and Organs - Threshold Doses for Tissue Reactions in a Radiation Protection Context. ICRP Publication 118. Ann ICRP. 2012; 41:1–322.16. Vaiserman AM. Radiation hormesis: historical perspective and implications for low-dose cancer risk assessment. Dose Response. 2010; 8:172–191.17. ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007; 37:1–332.18. International Commission on Radiological Protection. Radiation protection; recommendations. Adopted September 9, 1958. London: Pergamon;1959.19. Prekeges JL. Radiation hormesis, or, could all that radiation be good for us? J Nucl Med Technol. 2003; 31:11–17.20. Finch SC. Leukemia: lessons from the Japanese experience. Stem Cells. 1997; 15:Suppl 2. 135–139.21. Smith LG, Miller RC, Richards M, Brenner DJ, Hall EJ. Investigation of hypersensitivity to fractionated low-dose radiation exposure. Int J Radiat Oncol Biol Phys. 1999; 45:187–191.22. Krueger SA, Collis SJ, Joiner MC, Wilson GD, Marples B. Transition in survival from low-dose hyper-radiosensitivity to increased radioresistance is independent of activation of ATM Ser1981 activity. Int J Radiat Oncol Biol Phys. 2007; 69:1262–1271.23. Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005; 78:3–7.24. Redpath JL. Radiation-induced neoplastic transformation in vitro: evidence for a protective effect at low doses of low LET radiation. Cancer Metastasis Rev. 2004; 23:333–339.25. Azzam EI, de Toledo SM, Raaphorst GP, Mitchel RE. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat Res. 1996; 146:369–373.26. Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004; 73:39–85.27. Dikomey E, Brammer I. Relationship between cellular radiosensitivity and non-repaired double-strand breaks studied for different growth states, dose rates and plating conditions in a normal human fibroblast line. Int J Radiat Biol. 2000; 76:773–781.28. Zeng G, Day TK, Hooker AM, Blyth BJ, Bhat M, Tilley WD, Sykes PJ. Non-linear chromosomal inversion response in prostate after low dose X-radiation exposure. Mutat Res. 2006; 602:65–73.29. Columbano A, Endoh T, Denda A, Noguchi O, Nakae D, Hasegawa K, Ledda-Columbano GM, Zedda AI, Konishi Y. Effects of cell proliferation and cell death (apoptosis and necrosis) on the early stages of rat hepatocarcinogenesis. Carcinogenesis. 1996; 17:395–400.30. Löbrich M, Rief N, Kühne M, Heckmann M, Fleckenstein J, Rübe C, Uder M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A. 2005; 102:8984–8989.31. Mothersill C, Seymour CB. Radiation-induced bystander effects and the DNA paradigm: an “out of field” perspective. Mutat Res. 2006; 597:5–10.32. Schettino G, Folkard M, Michael BD, Prise KM. Low-dose binary behavior of bystander cell killing after microbeam irradiation of a single cell with focused c(k) x rays. Radiat Res. 2005; 163:332–336.33. Huang L, Kim PM, Nickoloff JA, Morgan WF. Targeted and nontargeted effects of low-dose ionizing radiation on delayed genomic instability in human cells. Cancer Res. 2007; 67:1099–1104.34. Okada M, Okabe A, Uchihori Y, Kitamura H, Sekine E, Ebisawa S, Suzuki M, Okayasu R. Single extreme low dose/low dose rate irradiation causes alteration in lifespan and genome instability in primary human cells. Br J Cancer. 2007; 96:1707–1710.35. Lefèvre SH, Vogt N, Dutrillaux AM, Chauveinc L, Stoppa-Lyonnet D, Doz F, Desjardins L, Dutrillaux B, Chevillard S, Malfoy B. Genome instability in secondary solid tumors developing after radiotherapy of bilateral retinoblastoma. Oncogene. 2001; 20:8092–8099.36. Salomaa S, Holmberg K, Lindholm C, Mustonen R, Tekkel M, Veidebaum T, Lambert B. Chromosomal instability in in vivo radiation exposed subjects. Int J Radiat Biol. 1998; 74:771–779.37. Tawn EJ, Whitehouse CA, Winther JF, Curwen GB, Rees GS, Stovall M, Olsen JH, Guldberg P, Rechnitzer C, Schrøder H, et al. Chromosome analysis in childhood cancer survivors and their offspring--no evidence for radiotherapy-induced persistent genomic instability. Mutat Res. 2005; 583:198–206.38. Nakanishi M, Tanaka K, Takahashi T, Kyo T, Dohy H, Fujiwara M, Kamada N. Microsatellite instability in acute myelocytic leukaemia developed from A-bomb survivors. Int J Radiat Biol. 2001; 77:687–694.39. Barquinero JF, Barrios L, Caballín MR, Miró R, Ribas M, Subias A, Egozcue J. Occupational exposure to radiation induces an adaptive response in human lymphocytes. Int J Radiat Biol. 1995; 67:187–191.40. Stoilov LM, Mullenders LH, Darroudi F, Natarajan AT. Adaptive response to DNA and chromosomal damage induced by X-rays in human blood lymphocytes. Mutagenesis. 2007; 22:117–122.41. Carbone MC, Pinto M, Antonelli F, Amicarelli F, Balata M, Belli M, Conti Devirgiliis L, Ioannucci L, Nisi S, Sapora O, et al. The Cosmic Silence experiment: on the putative adaptive role of environmental ionizing radiation. Radiat Environ Biophys. 2009; 48:189–196.42. Wolff S. Aspects of the adaptive response to very low doses of radiation and other agents. Mutat Res. 1996; 358:135–142.43. Calabrese EJ. Hormesis: from marginalization to mainstream: a case for hormesis as the default dose-response model in risk assessment. Toxicol Appl Pharmacol. 2004; 197:125–136.44. Seong KM, Kim CS, Seo SW, Jeon HY, Lee BS, Nam SY, Yang KH, Kim JY, Kim CS, Min KJ, et al. Genome-wide analysis of low-dose irradiated male Drosophila melanogaster with extended longevity. Biogerontology. 2011; 12:93–107.45. Calabrese EJ, Iavicoli I, Calabrese V. Hormesis: why it is important to biogerontologists. Biogerontology. 2012; 13:215–235.46. Seong KM, Kim CS, Lee BS, Nam SY, Yang KH, Kim JY, Park JJ, Min KJ, Jin YW. Low-dose radiation induces Drosophila innate immunity through Toll pathway activation. J Radiat Res (Tokyo). 2012; 53:242–249.47. Hatchwell E, Greally JM. The potential role of epigenomic dysregulation in complex human disease. Trends Genet. 2007; 23:588–595.48. Rugo RE, Schiestl RH. Increases in oxidative stress in the progeny of X-irradiated cells. Radiat Res. 2004; 162:416–425.49. de Toledo SM, Asaad N, Venkatachalam P, Li L, Howell RW, Spitz DR, Azzam EI. Adaptive responses to low-dose/low-dose-rate gamma rays in normal human fibroblasts: the role of growth architecture and oxidative metabolism. Radiat Res. 2006; 166:849–857.50. Marchetti F, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol. 2006; 82:605–639.51. Amundson SA. Functional genomics in radiation biology: a gateway to cellular systems-level studies. Radiat Environ Biophys. 2008; 47:25–31.52. Goldberg Z, Rocke DM, Schwietert C, Berglund SR, Santana A, Jones A, Lehmann J, Stern R, Lu R, Hartmann Siantar C. Human in vivo dose-response to controlled, low-dose low linear energy transfer ionizing radiation exposure. Clin Cancer Res. 2006; 12:3723–3729.53. Baverstock K, Rönkkö M. Epigenetic regulation of the mammalian cell. PLoS One. 2008; 3:e2290.54. Gilbert ES. Ionising radiation and cancer risks: what have we learned from epidemiology? Int J Radiat Biol. 2009; 85:467–482.55. ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007; 37:1–332.56. United Nations Scientific Committee on the Effects of Atomic Radiation. Report of the United Nations Scientific Committee on the effects of atomic radiation: fifty-seventh session, includes scientific report, summary of low-dose radiation effects on health. New York, NY: United Nations;2011.57. Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res. 2012; 177:229–243.58. Likhtarov I, Kovgan L, Vavilov S, Chepurny M, Ron E, Lubin J, Bouville A, Tronko N, Bogdanova T, Gulak L, et al. Post-Chernobyl thyroid cancers in Ukraine. Report 2: risk analysis. Radiat Res. 2006; 166:375–386.59. Jacob P, Bogdanova TI, Buglova E, Chepurniy M, Demidchik Y, Gavrilin Y, Kenigsberg J, Meckbach R, Schotola C, Shinkarev S, et al. Thyroid cancer risk in areas of Ukraine and Belarus affected by the Chernobyl accident. Radiat Res. 2006; 165:1–8.60. Cardis E, Howe G, Ron E, Bebeshko V, Bogdanova T, Bouville A, Carr Z, Chumak V, Davis S, Demidchik Y, et al. Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot. 2006; 26:127–140.61. UNSCEAR. Sources and effects of ionizing radiation vol. II: effects scientific annex D. Health effects due to radiation from the Chernobyl accident. UNSCEAR 2008 Report. New York, NY: United Nations;2011.62. Pukkala E, Kesminiene A, Poliakov S, Ryzhov A, Drozdovitch V, Kovgan L, Kyyrönen P, Malakhova IV, Gulak L, Cardis E. Breast cancer in Belarus and Ukraine after the Chernobyl accident. Int J Cancer. 2006; 119:651–658.63. International Consortium for Research on the Health Effects of Radiation Writing Committee and Study Team. Childhood leukaemia in Belarus, Russia, and Ukraine following the Chernobyl power station accident: results from an international collaborative population-based case-control study. Int J Epidemiol. 2006; 35:386–396.64. Ivanov EP, Tolochko GV, Shuvaeva LP, Ivanov VE, Iaroshevich RF, Becker S, Nekolla E, Kellerer AM. Infant leukemia in Belarus after the Chernobyl accident. Radiat Environ Biophys. 1998; 37:53–55.65. Kesminiene A, Cardis E, Tenet V, Ivanov VK, Kurtinaitis J, Malakhova I, Stengrevics A, Tekkel M. Studies of cancer risk among Chernobyl liquidators: materials and methods. J Radiol Prot. 2002; 22:A137–41.66. Kesminiene A, Evrard AS, Ivanov VK, Malakhova IV, Kurtinaitis J, Stengrevics A, Tekkel M, Anspaugh LR, Bouville A, Chekin S, et al. Risk of hematological malignancies among Chernobyl liquidators. Radiat Res. 2008; 170:721–735.67. Romanenko AY, Finch SC, Hatch M, Lubin JH, Bebeshko VG, Bazyka DA, Gudzenko N, Dyagil IS, Reiss RF, Bouville A, et al. The Ukrainian-American study of leukemia and related disorders among Chornobyl cleanup workers from Ukraine: III. Radiation risks. Radiat Res. 2008; 170:711–720.68. Ivanov VK. Late cancer and noncancer risks among Chernobyl emergency workers of Russia. Health Phys. 2007; 93:470–479.69. Cardis E, Hatch M. The Chernobyl accident--an epidemiological perspective. Clin Oncol (R Coll Radiol). 2011; 23:251–260.70. Hayashida N, Imaizumi M, Shimura H, Okubo N, Asari Y, Nigawara T, Midorikawa S, Kotani K, Nakaji S, Otsuru A, et al. Thyroid ultrasound findings in children from three Japanese prefectures: Aomori, Yamanashi and Nagasaki. PLoS One. 2013; 8:e83220.71. World Health Organization. Preliminary dose estimation from the nuclear accident after the 2011 Great East Japan Earthquake and Tsunami. Geneva: World Health Organization Press;2012.72. World Health Organization. Health risk assessment from the nuclear accident after the 2011 Great East Japan Earthquake and Tsunami, based on a preliminary dose estimation. Geneva: World Health Organization Press;2013.73. Harada KH, Niisoe T, Imanaka M, Takahashi T, Amako K, Fujii Y, Kanameishi M, Ohse K, Nakai Y, Nishikawa T, et al. Radiation dose rates now and in the future for residents neighboring restricted areas of the Fukushima Daiichi Nuclear Power Plant. Proc Natl Acad Sci U S A. 2014; 111:E914–23.74. Hatch MC, Wallenstein S, Beyea J, Nieves JW, Susser M. Cancer rates after the Three Mile Island nuclear accident and proximity of residence to the plant. Am J Public Health. 1991; 81:719–724.75. Wing S, Richardson D, Armstrong D, Crawford-Brown D. A reevaluation of cancer incidence near the Three Mile Island nuclear plant: the collision of evidence and assumptions. Environ Health Perspect. 1997; 105:52–57.76. Talbott EO, Youk AO, McHugh-Pemu KP, Zborowski JV. Long-term follow-up of the residents of the Three Mile Island accident area: 1979-1998. Environ Health Perspect. 2003; 111:341–348.77. Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat Res. 2007; 167:396–416.78. Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, Kuznetsova IS, Sokolnikov ME, Okatenko PV, Kreslov VV, et al. Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res. 2003; 159:787–798.79. Gilbert ES, Koshurnikova NA, Sokolnikov ME, Shilnikova NS, Preston DL, Ron E, Okatenko PV, Khokhryakov VF, Vasilenko EK, Miller S, et al. Lung cancer in Mayak workers. Radiat Res. 2004; 162:505–516.80. Hunter N, Kuznetsova IS, Labutina EV, Harrison JD. Solid cancer incidence other than lung, liver and bone in Mayak workers: 1948-2004. Br J Cancer. 2013; 109:1989–1996.81. Richardson DB, Wing S. Leukemia mortality among workers at the Savannah River Site. Am J Epidemiol. 2007; 166:1015–1022.82. Matanoski GM, Tonascia JA, Correa-Villaseñor A, Yates KC, Fink N, Elliott E, Sanders B, Lantry D. Cancer risks and low-level radiation in U.S. shipyard workers. J Radiat Res (Tokyo). 2008; 49:83–91.83. Metz-Flamant C, Laurent O, Samson E, Caër-Lorho S, Acker A, Hubert D, Richardson DB, Laurier D. Mortality associated with chronic external radiation exposure in the French combined cohort of nuclear workers. Occup Environ Med. 2013; 70:630–638.84. Akiba S, Mizuno S. The third analysis of cancer mortality among Japanese nuclear workers, 1991-2002: estimation of excess relative risk per radiation dose. J Radiol Prot. 2012; 32:73–83.85. Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure. J Radiol Prot. 2012; 32:N15–N19.86. Pukkala E, Auvinen A, Wahlberg G. Incidence of cancer among Finnish airline cabin attendants, 1967-92. BMJ. 1995; 311:649–652.87. Rafnsson V, Sulem P, Tulinius H, Hrafnkelsson J. Breast cancer risk in airline cabin attendants: a nested case-control study in Iceland. Occup Environ Med. 2003; 60:807–809.88. Reynolds P, Cone J, Layefsky M, Goldberg DE, Hurley S. Cancer incidence in California flight attendants (United States). Cancer Causes Control. 2002; 13:317–324.89. Wartenberg D, Stapleton CP. Risk of breast cancer is also increased among retired US female airline cabin attendants. BMJ. 1998; 316:1902.90. Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, Hammar N, Hrafnkelsson J, Kyyrönen P, Linnersjö A, et al. Incidence of cancer among Nordic airline pilots over five decades: occupational cohort study. BMJ. 2002; 325:567.91. Band PR, Spinelli JJ, Ng VT, Moody J, Gallagher RP. Mortality and cancer incidence in a cohort of commercial airline pilots. Aviat Space Environ Med. 1990; 61:299–302.92. Pinkerton LE, Waters MA, Hein MJ, Zivkovich Z, Schubauer-Berigan MK, Grajewski B. Cause-specific mortality among a cohort of U.S. flight attendants. Am J Ind Med. 2012; 55:25–36.93. Band PR, Le ND, Fang R, Deschamps M, Coldman AJ, Gallagher RP, Moody J. Cohort study of Air Canada pilots: mortality, cancer incidence, and leukemia risk. Am J Epidemiol. 1996; 143:137–143.94. Hammer GP, Auvinen A, De Stavola BL, Grajewski B, Gundestrup M, Haldorsen T, Hammar N, Lagorio S, Linnersjö A, Pinkerton L, et al. Mortality from cancer and other causes in commercial airline crews: a joint analysis of cohorts from 10 countries. Occup Environ Med. 2014; 71:313–322.95. Yong LC, Pinkerton LE, Yiin JH, Anderson JL, Deddens JA. Mortality among a cohort of U.S. commercial airline cockpit crew. Am J Ind Med. 2014; 57:906–914.96. Zeeb H, Blettner M, Langner I, Hammer GP, Ballard TJ, Santaquilani M, Gundestrup M, Storm H, Haldorsen T, Tveten U, et al. Mortality from cancer and other causes among airline cabin attendants in Europe: a collaborative cohort study in eight countries. Am J Epidemiol. 2003; 158:35–46.97. Muirhead CR, O’Hagan JA, Haylock RG, Phillipson MA, Willcock T, Berridge GL, Zhang W. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009; 100:206–212.98. Ahn YS, Park RM, Koh DH. Cancer admission and mortality in workers exposed to ionizing radiation in Korea. J Occup Environ Med. 2008; 50:791–803.99. Zablotska LB, Lane RS, Thompson PA. A reanalysis of cancer mortality in Canadian nuclear workers (1956-1994) based on revised exposure and cohort data. Br J Cancer. 2014; 110:214–223.100. Howe GR, Zablotska LB, Fix JJ, Egel J, Buchanan J. Analysis of the mortality experience amongst U.S. nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res. 2004; 162:517–526.101. Jeong M, Jin YW, Yang KH, Ahn YO, Cha CY. Radiation exposure and cancer incidence in a cohort of nuclear power industry workers in the Republic of Korea, 1992-2005. Radiat Environ Biophys. 2010; 49:47–55.102. Merzenich H, Hammer GP, Tröltzsch K, Ruecker K, Buncke J, Fehringer F, Blettner M. Mortality risk in a historical cohort of nuclear power plant workers in Germany: results from a second follow-up. Radiat Environ Biophys. 2014; 53:405–416.103. United Nations Scientific Committee on the Effects of Atomic Radiation. Annex B. Exposures from natural radiation sources. UNSCEAR 2000 Report. Sources and effects of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2000 Report to the General Assembly, with scientific annexes. Vol. I: Sources. New York, NY: United Nations;2001.104. Ghiassi-Nejad M, Mortazavi SM, Cameron JR, Niroomand-rad A, Karam PA. Very high background radiation areas of Ramsar, Iran: preliminary biological studies. Health Phys. 2002; 82:87–93.105. Hayata I, Wang C, Zhang W, Chen D, Minamihisamatsu M, Morishima H, Wei L, Sugahara T. Effect of high-level natural radiation on chromosomes of residents in southern China. Cytogenet Genome Res. 2004; 104:237–239.106. Ghiassi-Nejad M, Zakeri F, Assaei RG, Kariminia A. Long-term immune and cytogenetic effects of high level natural radiation on Ramsar inhabitants in Iran. J Environ Radioact. 2004; 74:107–116.107. Nair RR, Rajan B, Akiba S, Jayalekshmi P, Nair MK, Gangadharan P, Koga T, Morishima H, Nakamura S, Sugahara T. Background radiation and cancer incidence in Kerala, India-Karanagappally cohort study. Health Phys. 2009; 96:55–66.108. Tao Z, Akiba S, Zha Y, Sun Q, Zou J, Li J, Liu Y, Yuan Y, Tokonami S, Morishoma H, et al. Cancer and non-cancer mortality among Inhabitants in the high background radiation area of Yangjiang, China (1979-1998). Health Phys. 2012; 102:173–181.109. Zou J, Tao Z, Sun Q, Akiba S, Zha Y, Sugahara T, Wei L. Cancer and non-cancer epidemiological study in the high background radiation area of Yangjiang, China. Int Congr Ser. 2005; 1276:97–101.110. Wei LX, Zha YR, Tao ZF, He WH, Chen DQ, Yuan YL. Epidemiological investigation of radiological effects in high background radiation areas of Yangjiang, China. J Radiat Res (Tokyo). 1990; 31:119–136.111. Krestinina LY, Davis F, Ostroumova E, Epifanova S, Degteva M, Preston D, Akleyev A. Solid cancer incidence and low-dose-rate radiation exposures in the Techa River cohort: 1956 2002. Int J Epidemiol. 2007; 36:1038–1046.112. Krestinina L, Preston DL, Davis FG, Epifanova S, Ostroumova E, Ron E, Akleyev A. Leukemia incidence among people exposed to chronic radiation from the contaminated Techa River, 1953-2005. Radiat Environ Biophys. 2010; 49:195–201.113. Ostroumova E, Preston DL, Ron E, Krestinina L, Davis FG, Kossenko M, Akleyev A. Breast cancer incidence following low-dose rate environmental exposure: Techa River Cohort, 1956-2004. Br J Cancer. 2008; 99:1940–1945.114. Schonfeld SJ, Krestinina LY, Epifanova S, Degteva MO, Akleyev AV, Preston DL. Solid cancer mortality in the Techa river cohort (1950-2007). Radiat Res. 2013; 179:183–189.115. Standring WJ, Dowdall M, Strand P. Overview of dose assessment developments and the health of riverside residents close to the “Mayak” PA facilities, Russia. Int J Environ Res Public Health. 2009; 6:174–199.116. Hwang SL, Hwang JS, Yang YT, Hsieh WA, Chang TC, Guo HR, Tsai MH, Tang JL, Lin IF, Chang WP. Estimates of relative risks for cancers in a population after prolonged low-dose-rate radiation exposure: a follow-up assessment from 1983 to 2005. Radiat Res. 2008; 170:143–148.117. Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012; 380:499–505.118. Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, Giles GG, Wallace AB, Anderson PR, Guiver TA, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013; 346:f2360.119. de Gelder R, Draisma G, Heijnsdijk EA, de Koning HJ. Population-based mammography screening below age 50: balancing radiation-induced vs prevented breast cancer deaths. Br J Cancer. 2011; 104:1214–1220.120. Yaffe MJ, Mainprize JG. Risk of radiation-induced breast cancer from mammographic screening. Radiology. 2011; 258:98–105.121. Berrington de González A, Kim KP, Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Smith-Bindman R, Yee J, Kuntz KM, van Ballegooijen M, Zauber AG, et al. Radiation-related cancer risks from CT colonography screening: a risk-benefit analysis. AJR Am J Roentgenol. 2011; 196:816–823.122. Albert JM. Radiation risk from CT: implications for cancer screening. AJR Am J Roentgenol. 2013; 201:W81-7.123. Andrieu N, Easton DF, Chang-Claude J, Rookus MA, Brohet R, Cardis E, Antoniou AC, Wagner T, Simard J, Evans G, et al. Effect of chest X-rays on the risk of breast cancer among BRCA1/2 mutation carriers in the international BRCA1/2 carrier cohort study: a report from the EMBRACE, GENEPSO, GEO-HEBON, and IBCCS Collaborators’ Group. J Clin Oncol. 2006; 24:3361–3366.124. Gronwald J, Pijpe A, Byrski T, Huzarski T, Stawicka M, Cybulski C, van Leeuwen F, Lubiński J, Narod SA. Early radiation exposures and BRCA1-associated breast cancer in young women from Poland. Breast Cancer Res Treat. 2008; 112:581–584.125. Lecarpentier J, Noguès C, Mouret-Fourme E, Stoppa-Lyonnet D, Lasset C, Caron O, Fricker JP, Gladieff L, Faivre L, Sobol H, et al. Variation in breast cancer risk with mutation position, smoking, alcohol, and chest X-ray history, in the French National BRCA1/2 carrier cohort (GENEPSO). Breast Cancer Res Treat. 2011; 130:927–938.126. Pijpe A, Andrieu N, Easton DF, Kesminiene A, Cardis E, Noguès C, Gauthier-Villars M, Lasset C, Fricker JP, Peock S, et al. Exposure to diagnostic radiation and risk of breast cancer among carriers of BRCA1/2 mutations: retrospective cohort study (GENE-RAD-RISK). BMJ. 2012; 345:e5660.127. John EM, McGuire V, Thomas D, Haile R, Ozcelik H, Milne RL, Felberg A, West DW, Miron A, Knight JA, et al. Diagnostic chest X-rays and breast cancer risk before age 50 years for BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2013; 22:1547–1556.128. Goldfrank D, Chuai S, Bernstein JL, Ramon Y, Cajal T, Lee JB, Alonso MC, Diez O, Baiget M, Kauff ND, et al. Effect of mammography on breast cancer risk in women with mutations in BRCA1 or BRCA2. Cancer Epidemiol Biomarkers Prev. 2006; 15:2311–2313.129. Narod SA, Lubinski J, Ghadirian P, Lynch HT, Moller P, Foulkes WD, Rosen B, Kim-Sing C, Isaacs C, Domchek S, et al. Screening mammography and risk of breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet Oncol. 2006; 7:402–406.130. National Research Council (US) Committee on an Assessment of CDC Radiation Studies. Radiation dose reconstruction for epidemiologic uses. Washington, D.C.: National Academy Press;1995.131. Gilbert ES. The impact of dosimetry uncertainties on dose-response analyses. Health Phys. 2009; 97:487–492.132. National Council on Radiation Protection and Measurements. Uncertainties in the estimation of radiation risks and probability of disease causation, NCRP Report No. 171. Bethesda, MD: National Council on Radiation Protection and Measurements;2012.133. Committee on Research Directions in Human Biological Effects of Low-Level Ionizing Radiation. Institute of Medicine (US), Board on the Health of Select Populations. National Research Council (US), Nuclear and Radiation Studies Board. Research on health effects of low-level ionizing radiation exposure: opportunities for the Armed Forces Radiobiology Research Institute. Washington, D.C.: National Academies Press;2014.134. Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, Hamra GB, Haylock R, Laurier D, Moissonnier M, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2015; 2:e276–81.135. Cook R, Calabrese EJ. The importance of hormesis to public health. Cien Saude Colet. 2007; 12:955–963.136. Calabrese EJ, Shamoun DY, Hanekamp JC. Cancer risk assessment: Optimizing human health through linear dose-response models. Food Chem Toxicol. 2015; 81:137–140.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The health effects of low-dose radiation exposure
- Low-Dose Radiation-Induced Effects on Cognitive Function
- Genetic radiation risks: a neglected topic in the low dose debate
- Assessment of the Glycophorin A Mutant Assay as a Biologic Marker for Low Dose Radiation Exposure
- Radiation-Induced Neovascular Glaucoma: Dose and Volume Issues