Investig Clin Urol.
2016 Mar;57(2):84-93. 10.4111/icu.2016.57.2.84.
Persistence and compliance with medication management in the treatment of overactive bladder
- Affiliations
-
- 1Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ksleedr@skku.edu
- 2Department of Medical Device Management & Research, SAIHST, Sungkyunkwan University, Seoul, Korea.
- KMID: 2363129
- DOI: http://doi.org/10.4111/icu.2016.57.2.84
Abstract
- Overactive bladder (OAB) is a common and chronic condition that impacts patients' daily activities and quality of life. Pharmaco-therapy for OAB is a mainstay of treatment. Antimuscarinics and beta3-adrenoceptor agonists are the two major classes of oral pharmacotherapy and have similar efficacy for treating the symptoms of OAB. Owing to the chronic nature of OAB, long-term use of medication is essential for OAB symptom control and positive health outcomes. However, many patients elect to stop their medications during the treatment period. Unmet expectations of treatment and side effects seem to be the major factors for discontinuing OAB pharmacotherapy. Furthermore, the short- and long-term persistence and compliance with medication management are markedly worse in OAB than in other chronic medical conditions. Improvement in persistence and compliance with OAB pharmacotherapy is a hot topic in OAB treatment and should be an important goal in the treatment of OAB. Effective strategies should be identified to improve persistence and compliance. In this review, we outline what is known about persistence and compliance and the factors affecting persistence with pharmacotherapy in patients with OAB.
Keyword
MeSH Terms
-
Adrenergic beta-3 Receptor Agonists/administration & dosage/therapeutic use
Humans
*Medication Adherence
Muscarinic Antagonists/*administration & dosage/therapeutic use
Terminology as Topic
Urinary Bladder, Overactive/*drug therapy
Urological Agents/*administration & dosage/therapeutic use
Adrenergic beta-3 Receptor Agonists
Muscarinic Antagonists
Urological Agents
Figure
Cited by 2 articles
-
Evaluation of the incidence and risk factors associated with persistent frequency in interstitial cystitis/bladder pain syndrome and the efficacy of antimuscarinic treatment
Aram Kim, Kyeong-Ok Hoe, Jung Hyun Shin, Myung-Soo Choo
Investig Clin Urol. 2017;58(5):353-358. doi: 10.4111/icu.2017.58.5.353.Patient utilization survey of mirabegron prescribed for overactive bladder
Päivi Rahkola-Soisalo, Marcin Balcerzak, Jarno Ruotsalainen, Tomi S. Mikkola
Investig Clin Urol. 2019;60(2):114-119. doi: 10.4111/icu.2019.60.2.114.
Reference
-
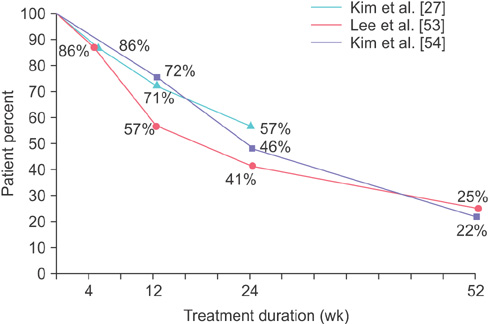
1. Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010; 21:5–26.2. Tikkinen KA, Tammela TL, Rissanen AM, Valpas A, Huhtala H, Auvinen A. Is the prevalence of overactive bladder overestimated? A population-based study in Finland. PLoS One. 2007; 7(2):e195.3. Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006; 50:1306–1314.4. Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003; 20:327–336.5. Homma Y, Yamaguchi O, Hayashi K. Neurogenic Bladder Society Committee. An epidemiological survey of overactive bladder symptoms in Japan. BJU Int. 2005; 96:1314–1318.6. Lee YS, Lee KS, Jung JH, Han DH, Oh SJ, Seo JT, et al. Prevalence of overactive bladder, urinary incontinence, and lower urinary tract symptoms: results of Korean EPIC study. World J Urol. 2011; 29:185–190.7. Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. 2008; 54:543–562.8. Brown JS, McGhan WF, Chokroverty S. Comorbidities associated with overactive bladder. Am J Manag Care. 2000; 6:11 Suppl. S574–S579.9. Wagner TH, Hu TW, Bentkover J, LeBlanc K, Stewart W, Corey R, et al. Health-related consequences of overactive bladder. Am J Manag Care. 2002; 8:19 Suppl. S598–S607.10. Irwin DE, Milsom I, Kopp Z, Abrams P, Cardozo L. Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. BJU Int. 2006; 97:96–100.11. Coyne KS, Sexton CC, Kopp ZS, Ebel-Bitoun C, Milsom I, Chapple C. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden: results from EpiLUTS. BJU Int. 2011; 108:1459–1471.12. Thom DH, Haan MN, Van Den Eeden SK. Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age Ageing. 1997; 26:367–374.13. Lee YI, Kim JW, Bae SR, Paick SH, Kim KW, Kim HG, et al. Effect of urgency symptoms on the risk of depression in community-dwelling elderly men. Korean J Urol. 2013; 54:762–766.14. Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012; 188:6 Suppl. 2455–2463.15. Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollen-dorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008; 11:44–47.16. Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the california medicaid program. Value Health. 2005; 8:495–505.17. Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Cardozo L, Subramanian D, et al. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract. 2011; 65:567–585.18. Lutfey KE, Wishner WJ. Beyond "compliance" is "adherence". Improving the prospect of diabetes care. Diabetes Care. 1999; 22:635–639.19. Feinstein AR. On white-coat effects and the electronic monitoring of compliance. Arch Intern Med. 1990; 150:1377–1378.20. Irwin DE, Milsom I, Chancellor MB, Kopp Z, Guan Z. Dynamic progression of overactive bladder and urinary incontinence symptoms: a systematic review. Eur Urol. 2010; 58:532–543.21. Malmsten UG, Molander U, Peeker R, Irwin DE, Milsom I. Urinary incontinence, overactive bladder, and other lower urinary tract symptoms: a longitudinal population-based survey in men aged 45-103 years. Eur Urol. 2010; 58:149–156.22. Wennberg AL, Molander U, Fall M, Edlund C, Peeker R, Milsom I. A longitudinal population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in women. Eur Urol. 2009; 55:783–791.23. Abrams P, Kelleher CJ, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care. 2000; 6:11 Suppl. S580–S590.24. Rogers RG, Omotosho T, Bachmann G, Sun F, Morrow JD. Continued symptom improvement in sexually active women with overactive bladder and urgency urinary incontinence treated with tolterodine ER for 6 months. Int Urogynecol J Pelvic Floor Dysfunct. 2009; 20:381–385.25. Choo MS, Song C, Kim JH, Choi JB, Lee JY, Chung BS, et al. Changes in overactive bladder symptoms after discontinuation of successful 3-month treatment with an antimuscarinic agent: a prospective trial. J Urol. 2005; 174:201–204.26. Lee YS, Choo MS, Lee JY, Oh SJ, Lee KS. Symptom change after discontinuation of successful antimuscarinic treatment in patients with overactive bladder symptoms: a randomised, multicentre trial. Int J Clin Pract. 2011; 65:997–1004.27. Kim TH, Choo MS, Kim YJ, Koh H, Lee KS. Drug persistence and compliance affect patient-reported outcomes in overactive bladder syndrome. Qual Life Res. 2015; 12. 24. DOI: 10.1007/s11136-015-1216-z.28. Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: a comprehensive review. Adv Ther. 2005; 22:313–356.29. Schabert VF, Bavendam T, Goldberg EL, Trocio JN, Brubaker L. Challenges for managing overactive bladder and guidance for patient support. Am J Manag Care. 2009; 15:4 Suppl. S118–S122.30. Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009; 15:728–740.31. Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol. 2006; 175(3 Pt 1):999–1004.32. Rogers R, Bachmann G, Jumadilova Z, Sun F, Morrow JD, Guan Z, et al. Efficacy of tolterodine on overactive bladder symptoms and sexual and emotional quality of life in sexually active women. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:1551–1557.33. Cardozo L, Lisec M, Millard R, van Vierssen Trip O, Kuzmin I, Drogendijk TE, et al. Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004; 172(5 Pt 1):1919–1924.34. Nitti VW, Dmochowski R, Sand PK, Forst HT, Haag-Molken-teller C, Massow U, et al. Efficacy, safety and tolerability of fesoterodine for overactive bladder syndrome. J Urol. 2007; 178:2488–2494.35. Drutz HP, Appell RA, Gleason D, Klimberg I, Radomski S. Clinical efficacy and safety of tolterodine compared to oxybutynin and placebo in patients with overactive bladder. Int Urogynecol J Pelvic Floor Dysfunct. 1999; 10:283–289.36. Homma Y, Paick JS, Lee JG, Kawabe K. Japanese and Korean Tolterodine Study Group. Clinical efficacy and tolerability of extended-release tolterodine and immediate-release oxybutynin in Japanese and Korean patients with an overactive bladder: a randomized, placebo-controlled trial. BJU Int. 2003; 92:741–747.37. Armstrong RB, Luber KM, Peters KM. Comparison of dry mouth in women treated with extended-release formulations of oxybutynin or tolterodine for overactive bladder. Int Urol Nephrol. 2005; 37:247–252.38. Chapple CR, Rechberger T, Al-Shukri S, Meffan P, Everaert K, Huang M, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004; 93:303–310.39. Chapple CR, Martinez-Garcia R, Selvaggi L, Toozs-Hobson P, Warnack W, Drogendijk T, et al. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol. 2005; 48:464–470.40. Chapple C, Van Kerrebroeck P, Tubaro A, Haag-Molkenteller C, Forst HT, Massow U, et al. Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol. 2007; 52:1204–1212.41. Giannitsas K, Perimenis P, Athanasopoulos A, Gyftopoulos K, Nikiforidis G, Barbalias G. Comparison of the efficacy of tolterodine and oxybutynin in different urodynamic severity grades of idiopathic detrusor overactivity. Eur Urol. 2004; 46:776–782.42. Sand PK, Miklos J, Ritter H, Appell R. A comparison of extended-release oxybutynin and tolterodine for treatment of overactive bladder in women. Int Urogynecol J Pelvic Floor Dysfunct. 2004; 15:243–248.43. Salvatore S, Khullar V, Cardozo L, Milani R, Athanasiou S, Kelleher C. Long-term prospective randomized study comparing two different regimens of oxybutynin as a treatment for detrusor overactivity. Eur J Obstet Gynecol Reprod Biol. 2005; 119:237–241.44. Abrams P, Malone-Lee J, Jacquetin B, Wyndaele JJ, Tammela T, Jonas U, et al. Twelve-month treatment of overactive bladder: efficacy and tolerability of tolterodine. Drugs Aging. 2001; 18:551–560.45. Desgagne A, LeLorier J. Incontinence drug utilization patterns in Québec, Canada. Value Health. 1999; 2:452–458.46. Malone DC, Okano GJ. Treatment of urge incontinence in Veterans Affairs medical centers. Clin Ther. 1999; 21:867–877.47. Shaya FT, Blume S, Gu A, Zyczynski T, Jumadilova Z. Persistence with overactive bladder pharmacotherapy in a Medicaid population. Am J Manag Care. 2005; 11:4 Suppl. S121–S129.48. Gomes T, Juurlink DN, Mamdani MM. Comparative adherence to oxybutynin or tolterodine among older patients. Eur J Clin Pharmacol. 2012; 68:97–99.49. Wagg A, Compion G, Fahey A, Siddiqui E. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int. 2012; 110:1767–1774.50. Dmochowski RR, Newman DK. Impact of overactive bladder on women in the United States: results of a national survey. Curr Med Res Opin. 2007; 23:65–76.51. Diokno A, Sand P, Labasky R, Sieber P, Antoci J, Leach G, et al. Long-term safety of extended-release oxybutynin chloride in a community-dwelling population of participants with overactive bladder: a one-year study. Int Urol Nephrol. 2002; 34:43–49.52. Homma Y, Yamaguchi O. Long-term safety, tolerability, and efficacy of the novel anti-muscarinic agent imidafenacin in Japanese patients with overactive bladder. Int J Urol. 2008; 15:986–991.53. Lee YS, Lee KS, Kim JC, Hong S, Chung BH, Kim CS, et al. Persistence with solifenacin add-on therapy in men with benign prostate obstruction and residual symptoms of overactive bladder after tamsulosin monotherapy. Int J Clin Pract. 2014; 68:1496–1502.54. Kim TH, You HW, Park JH, Lee JG, Choo MS, Park WH. Persistence of solifenacin therapy in patients with overactive bladder in the clinical setting: a prospective, multicenter, observational study. Int J Clin Pract Forthcoming 2016.55. Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014; 33:17–30.56. Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014; 65:755–765.57. Pindoria N, Malde S, Nowers J, Taylor C, Kelleher C, Sahai A. Persistence with mirabegron therapy for overactive bladder: A real life experience. Neurourol Urodyn. 2015; 12. 15. DOI: 10.1002/nau.22943.58. Wagg A, Franks B, Ramos B, Berner T. Persistence and adherence with the new beta-3 receptor agonist, mirabegron, versus antimuscarinics in overactive bladder: early experience in Canada. Can Urol Assoc J. 2015; 9:343–350.59. D'Souza AO, Smith MJ, Miller LA, Doyle J, Ariely R. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J Manag Care Pharm. 2008; 14:291–301.60. Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010; 105:1276–1282.61. Klutke CG, Burgio KL, Wyman JF, Guan Z, Sun F, Berriman S, et al. Combined effects of behavioral intervention and tolterodine in patients dissatisfied with overactive bladder medication. J Urol. 2009; 181:2599–2607.62. Wyman JF, Burgio KL, Newman DK. Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency urinary incontinence. Int J Clin Pract. 2009; 63:1177–1191.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Analysis of Persistent Overactive Bladder Syndrome after Sling Surgery in Female Stress Urinary Incontinence
- Factors Associated With Compliance and Persistence With Pharmacotherapy in Patients With Osteoporosis: A Nationwide Cohort Study in Korea
- Overactive Bladder
- Medical Treatment of Overactive Bladder
- New Frontiers in the Treatment of Overactive Bladder