J Periodontal Implant Sci.
2016 Dec;46(6):362-371. 10.5051/jpis.2016.46.6.362.
Biocompatibility study of lithium disilicate and zirconium oxide ceramics for esthetic dental abutments
- Affiliations
-
- 1Laboratory of Biomaterials and Bone Site Inflammation, University of Reims Champagne-Ardenne, Reims, France. celine.brunot-gohin@univ-reims.fr
- 2Laboratory of Biomechanics and Bioengineering, Research Center of Royallieu, University of Technology of Compiègne, Sorbonne Universities, Compiègne, France.
- 3Laboratory of Integrated Renewable Matter Transformations, Research Center of Royallieu, University of Technology of Compiègne, Sorbonne Universities, Compiègne, France.
- 4University of Reims Champagne-Ardenne, Faculty of Odontology, Reims, France.
- 5Tufts University School of Dental Medicine, Boston, MA, USA.
- KMID: 2362902
- DOI: http://doi.org/10.5051/jpis.2016.46.6.362
Abstract
- PURPOSE
The increasing demand for esthetically pleasing results has contributed to the use of ceramics for dental implant abutments. The aim of this study was to compare the biological response of epithelial tissue cultivated on lithium disilicate (LSâ‚‚) and zirconium oxide (ZrOâ‚‚) ceramics. Understanding the relevant physicochemical and mechanical properties of these ceramics will help identify the optimal material for facilitating gingival wound closure.
METHODS
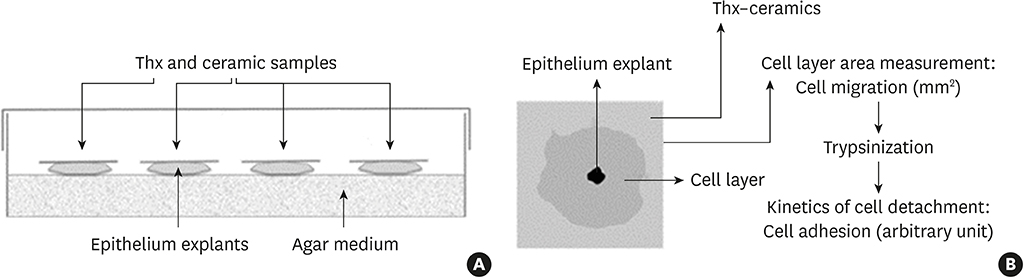
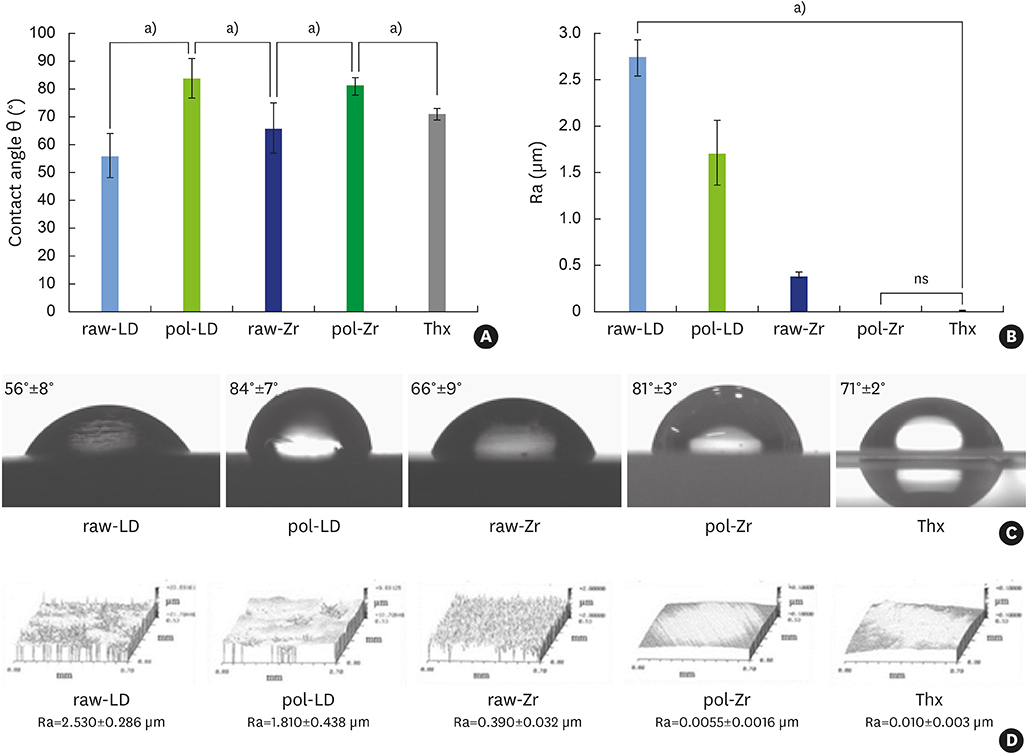
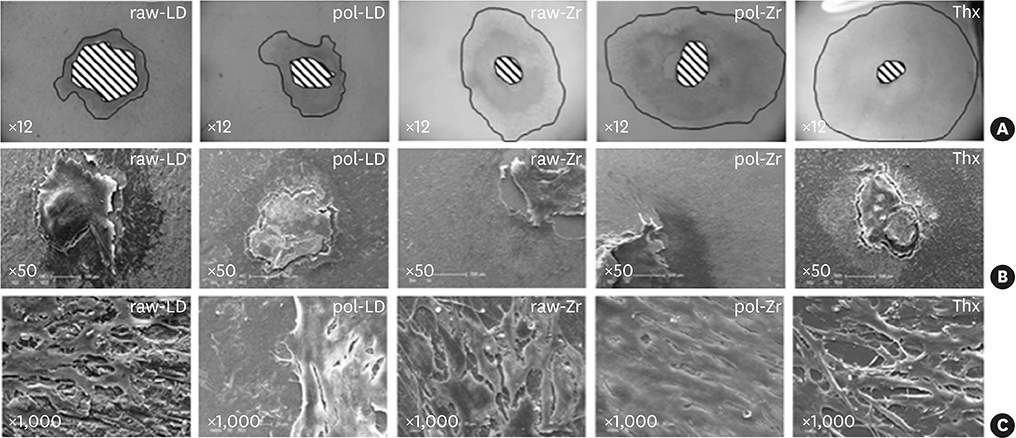
Both biomaterials were prepared with 2 different surface treatments: raw and polished. Their physicochemical characteristics were analyzed by contact angle measurements, scanning white-light interferometry, and scanning electron microscopy. An organotypic culture was then performed using a chicken epithelium model to simulate peri-implant soft tissue. We measured the contact angle, hydrophobicity, and roughness of the materials as well as the tissue behavior at their surfaces (cell migration and cell adhesion).
RESULTS
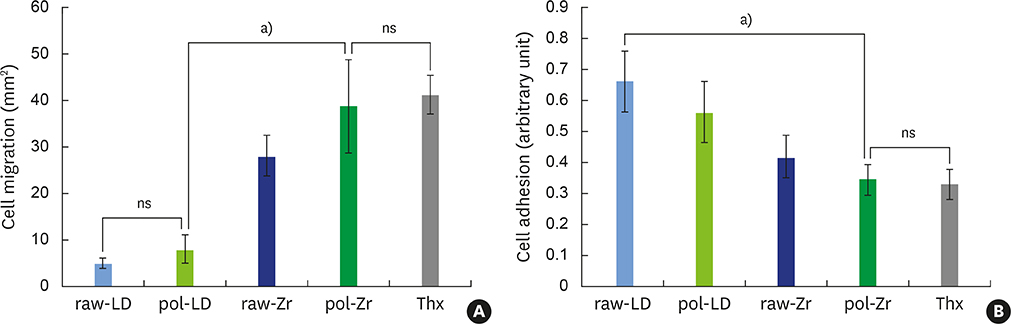
The best cell migration was observed on ZrOâ‚‚ ceramic. Cell adhesion was also drastically lower on the polished ZrOâ‚‚ ceramic than on both the raw and polished LS2. Evaluating various surface topographies of LS2 showed that increasing surface roughness improved cell adhesion, leading to an increase of up to 13%.
CONCLUSIONS
Our results demonstrate that a biomaterial, here LS2, can be modified using simple surface changes in order to finely modulate soft tissue adhesion. Strong adhesion at the abutment associated with weak migration assists in gingival wound healing. On the same material, polishing can reduce cell adhesion without drastically modifying cell migration. A comparison of LS2 and ZrO2 ceramic showed that LS2 was more conducive to creating varying tissue reactions. Our results can help dental surgeons to choose, especially for esthetic implant abutments, the most appropriate biomaterial as well as the most appropriate surface treatment to use in accordance with specific clinical dental applications.
MeSH Terms
-
Biocompatible Materials
Cell Adhesion
Cell Movement
Ceramics*
Chickens
Dental Abutments*
Dental Implants
Embryo Culture Techniques
Epithelium
Esthetics, Dental
Hydrophobic and Hydrophilic Interactions
Interferometry
Lithium*
Microscopy, Electron, Scanning
Surgeons
Tissue Adhesions
Wound Healing
Wounds and Injuries
Zirconium*
Biocompatible Materials
Dental Implants
Lithium
Zirconium
Figure
Reference
-
1. Valenti M, Valenti A. Retrospective survival analysis of 261 lithium disilicate crowns in a private general practice. Quintessence Int. 2009; 40:573–579.2. Gehrt M, Wolfart S, Rafai N, Reich S, Edelhoff D. Clinical results of lithium-disilicate crowns after up to 9 years of service. Clin Oral Investig. 2013; 17:275–284.
Article3. Herrguth M, Wichmann M, Reich S. The aesthetics of all-ceramic veneered and monolithic CAD/CAM crowns. J Oral Rehabil. 2005; 32:747–752.
Article4. Bengazi F, Wennström JL, Lekholm U. Recession of the soft tissue margin at oral implants. A 2-year longitudinal prospective study. Clin Oral Implants Res. 1996; 7:303–310.
Article5. Bressan E, Paniz G, Lops D, Corazza B, Romeo E, Favero G. Influence of abutment material on the gingival color of implant-supported all-ceramic restorations: a prospective multicenter study. Clin Oral Implants Res. 2011; 22:631–637.
Article6. Ekfeldt A, Fürst B, Carlsson GE. Zirconia abutments for single-tooth implant restorations: a retrospective and clinical follow-up study. Clin Oral Implants Res. 2011; 22:1308–1314.
Article7. Grunder U, Gracis S, Capelli M. Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent. 2005; 25:113–119.8. Guess PC, Zavanelli RA, Silva NR, Bonfante EA, Coelho PG, Thompson VP. Monolithic CAD/CAM lithium disilicate versus veneered Y-TZP crowns: comparison of failure modes and reliability after fatigue. Int J Prosthodont. 2010; 23:434–442.9. Van Dooren E, Calamita M, Calgaro M, Coachman C, Ferencz JL, Pinho C, et al. Mechanical, biological and clinical aspects of zirconia implants. Eur J Esthet Dent. 2012; 7:396–417.10. Wittneben JG, Wright RF, Weber HP, Gallucci GO. A systematic review of the clinical performance of CAD/CAM single-tooth restorations. Int J Prosthodont. 2009; 22:466–471.11. Grundke K, Bogumil T, Werner C, Janke A, Pöschel K, Jacobasch HJ. Liquid-fluid contact angle measurements on hydrophilic cellulosic materials. Colloids Surf A Physicochem Eng Asp. 1996; 116:79–91.
Article12. de Groot P, Deck L. Surface profiling by frequency-domain analysis of white light interferograms. Proc SPIE. 1994; 2248:101–104.13. Rajaraman R, Rounds DE, Yen SP, Rembaum A. A scanning electron microscope study of cell adhesion and spreading in vitro . Exp Cell Res. 1974; 88:327–339.
Article14. Duval JL, Letort M, Sigot-Luizard MF. Comparative assessment of cell/substratum static adhesion using an in vitro organ culture method and computerized analysis system. Biomaterials. 1988; 9:155–161.
Article15. Sigot-Luizard MF, Lanfranchi M, Duval JL, Benslimane S, Sigot M, Guidoin RG, et al. The cytocompatibility of compound polyester-protein surfaces using an in vitro technique. In Vitro Cell Dev Biol. 1986; 22:234–240.
Article16. Abramoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004; 11:36–42.17. Josset Y. Oum’Hamed Z, Zarrinpour A, Lorenzato M, Adnet JJ, Laurent-Maquin D. In vitro reactions of human osteoblasts in culture with zirconia and alumina ceramics. J Biomed Mater Res. 1999; 47:481–493.
Article18. Covacci V, Bruzzese N, Maccauro G, Andreassi C, Ricci GA, Piconi C, et al. In vitro evaluation of the mutagenic and carcinogenic power of high purity zirconia ceramic. Biomaterials. 1999; 20:371–376.
Article19. Warashina H, Sakano S, Kitamura S, Yamauchi KI, Yamaguchi J, Ishiguro N, et al. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials. 2003; 24:3655–3661.
Article20. van Brakel R, Meijer GJ, Verhoeven JW, Jansen J, de Putter C, Cune MS. Soft tissue response to zirconia and titanium implant abutments: an in vivo within-subject comparison. J Clin Periodontol. 2012; 39:995–1001.
Article21. De Sanctis M, Baldini N, Vignoletti F. Soft tissue management around teeth and implants. J Parodontol Implantol Oral. 2010; 29:245–269.22. Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002; 17:793–798.23. Brunot-Gohin C, Duval JL, Azogui EE, Jannetta R, Pezron I, Laurent-Maquin D, et al. Soft tissue adhesion of polished versus glazed lithium disilicate ceramic for dental applications. Dent Mater. 2013; 29:e205–12.
Article24. Müller C, Lüders A, Hoth-Hannig W, Hannig M, Ziegler C. Initial bioadhesion on dental materials as a function of contact time, pH, surface wettability, and isoelectric point. Langmuir. 2010; 26:4136–4141.
Article25. Bolortuya G, Ebihara A, Ichinose S, Watanabe S, Anjo T, Kokuzawa C, et al. Effects of dentin surface modifications treated with Er:YAG and Nd:YAG laser irradiation on fibroblast cell adhesion. Photomed Laser Surg. 2012; 30:63–70.
Article26. Duval JL, Dinis T, Vidal G, Vigneron P, Kaplan DL, Egles C. Organotypic culture to assess cell adhesion, growth and alignment of different organs on silk fibroin. J Tissue Eng Regen Med. Forthcoming. 2014.
Article27. Messer RL, Lockwood PE, Wataha JC, Lewis JB, Norris S, Bouillaguet S. In vitro cytotoxicity of traditional versus contemporary dental ceramics. J Prosthet Dent. 2003; 90:452–458.
Article28. Brackett MG, Lockwood PE, Messer RL, Lewis JB, Bouillaguet S, Wataha JC. In vitro cytotoxic response to lithium disilicate dental ceramics. Dent Mater. 2008; 24:450–456.29. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004; 75:292–296.
Article30. Rompen E, Raepsaet N, Domken O, Touati B, Van Dooren E. Soft tissue stability at the facial aspect of gingivally converging abutments in the esthetic zone: a pilot clinical study. J Prosthet Dent. 2007; 97:S119–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative analysis of transmittance for different types of commercially available zirconia and lithium disilicate materials
- Mechanical Properties Of Reused Lithium Disilicate Glass-Ceramic Of Ips Empress 2 System
- Esthetic restoration of anterior dentition using Empress 2 system (A clinical report)
- Comparison of the translucency of shaded zirconia all-ceramic systems
- A prospective clinical of lithium disilicate pressed zirconia and monolithic zirconia in posterior implant-supported prostheses: A 24-month follow-up