Immune Netw.
2016 Dec;16(6):317-321. 10.4110/in.2016.16.6.317.
Neutrophil Extravasation Cascade: What Can We Learn from Two-photon Intravital Imaging?
- Affiliations
-
- 1Department of Anatomy, Yonsei University College of Medicine, Seoul 03722, Korea. ymhyun@yuhs.ac
- KMID: 2362795
- DOI: http://doi.org/10.4110/in.2016.16.6.317
Abstract
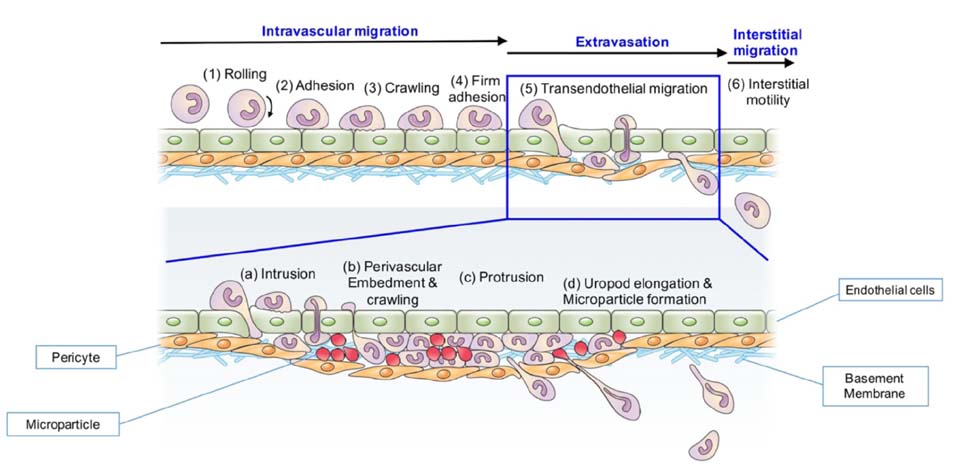
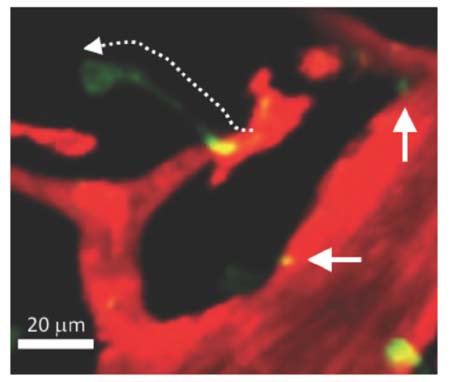
- Immune cells (leukocytes or white blood cells) move actively and sensitively based on body conditions. Despite their important role as protectors inside the body, it is difficult to directly observe the spatiotemporal momentum of leukocytes. With advances in imaging technology, the introduction of two-photon microscopy has enabled researchers to look deeper inside tissues in a three-dimensional manner. In observations of immune cell movement along the blood vessel, vascular permeability and innate immune cell movements remain unclear. Here, we describe the neutrophil extravasation cascade, which were observed using a two-photon intravital imaging technique. We also provide evidence for novel mechanisms such as neutrophil body extension and microparticle formation as well as their biological roles during migration.
Keyword
Figure
Reference
-
1. Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002; 2:872–880.
Article2. Rubart M. Two-photon microscopy of cells and tissue. Circ Res. 2004; 95:1154–1166.
Article3. Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005; 2:932–940.
Article4. Stephens DJ, Allan VJ. Light microscopy techniques for live cell imaging. Science. 2003; 300:82–86.
Article5. Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011; 12:761–769.
Article6. McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010; 330:362–366.
Article7. Ishii T, Ishii M. Intravital two-photon imaging: a versatile tool for dissecting the immune system. Ann Rheum Dis. 2011; 70:Suppl 1. i113–i115.
Article8. Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014; 505:223–228.
Article9. Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006; 203:2569–2575.
Article10. Voisin MB, Nourshargh S. Neutrophil transmigration: emergence of an adhesive cascade within venular walls. J Innate Immun. 2013; 5:336–347.
Article11. Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998; 53:637–644.
Article12. Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012; 45:327–347.
Article13. Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, Fowell DJ, Waugh RE, Sarelius IH, Kim M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med. 2012; 209:1349–1362.
Article14. Lerman YV, Lim K, Hyun YM, Falkner KL, Yang H, Pietropaoli AP, Sonnenberg A, Sarangi PP, Kim M. Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin alpha3beta1-dependent. Blood. 2014; 24:3515–3523.15. Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003; 9:263–268.
Article16. Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009; 122:215–225.
Article17. DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009; 30:547–556.
Article18. He P. Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled? Cardiovasc Res. 2010; 87:281–290.
Article19. Wedmore CV, Williams TJ. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981; 289:646–650.
Article20. Lim K, Sumagin R, Hyun YM. Extravasating neutrophil-derived microparticles preserve vascular barrier function in inflamed tissue. Immune Netw. 2013; 13:102–106.
Article21. Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002; 99:2703–2711.
Article22. Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, Dulak J, Jozkowicz A, Grant MB, Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007; 204:605–618.
Article23. Pi X, Wu Y, Ferguson JE III, Portbury AL, Patterson C. SDF-1alpha stimulates JNK3 activity via eNOS-dependent nitrosylation of MKP7 to enhance endothelial migration. Proc Natl Acad Sci U S A. 2009; 106:5675–5680.
Article24. Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, Topham DJ, Kim M. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science. 2015; 349:aaa4352.25. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011; 11:519–531.
Article26. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010; 327:291–295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Real-time observation of neutrophil extracellular trap formation in the inflamed mouse brain via two-photon intravital imaging
- Intravital Laser-scanning Two-photon and Confocal Microscopy for Biomedical Research
- Two-photon intravital imaging of leukocyte migration during inflammation in the respiratory system
- Intravital Two-photon Imaging of Dynamic Alteration of Hepatic Lipid Droplets in Fasted and Refed State
- Extravasating Neutrophil-derived Microparticles Preserve Vascular Barrier Function in Inflamed Tissue