Acute Crit Care.
2019 May;34(2):101-107. 10.4266/acc.2019.00542.
Two-photon intravital imaging of leukocyte migration during inflammation in the respiratory system
- Affiliations
-
- 1Department of Anatomy and Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. ymhyun@yuhs.ac
- KMID: 2449363
- DOI: http://doi.org/10.4266/acc.2019.00542
Abstract
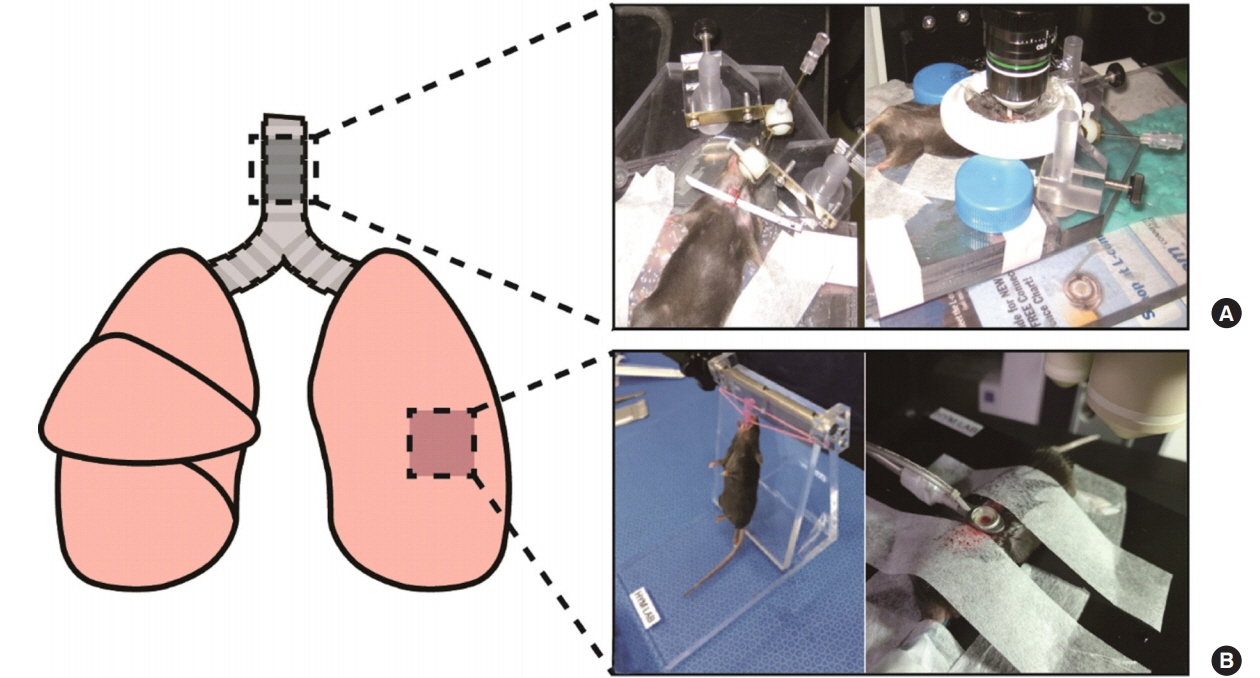
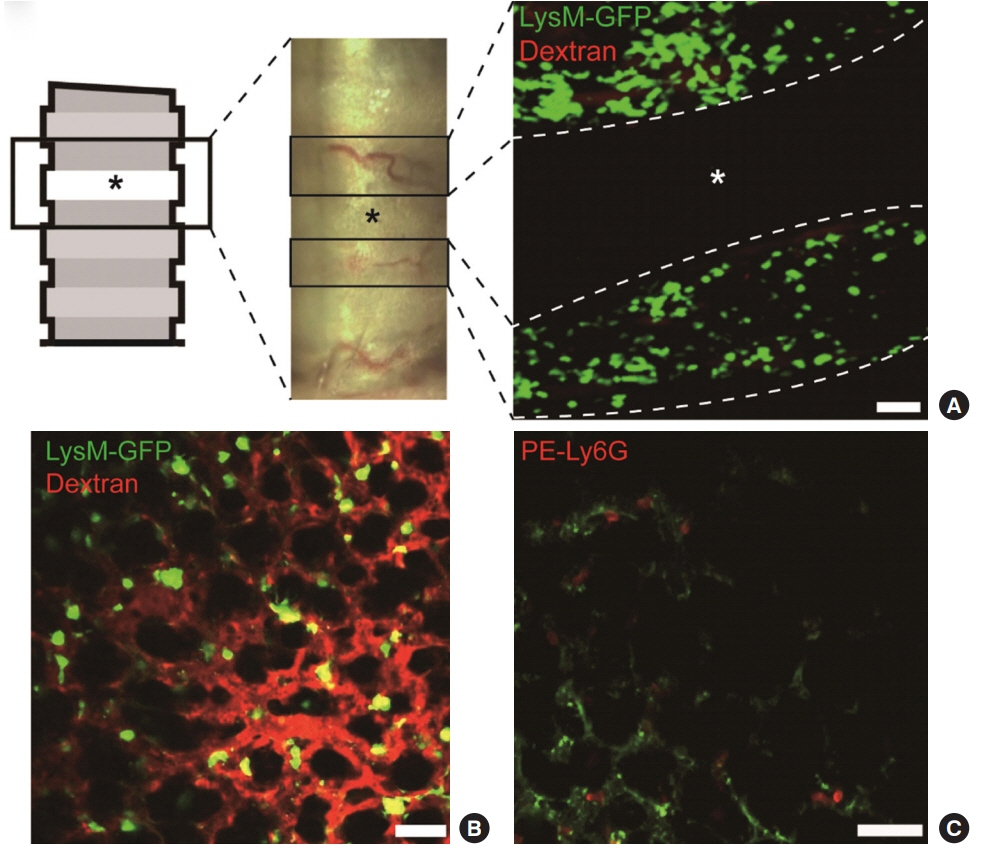
- Two-photon intravital imaging is a powerful method by which researchers are able to directly observe biological phenomena in live organisms. Researchers in various biomedical research fields have applied two-photon imaging to a variety of target organs by utilizing this technology's ability to penetrate to significant depths with minimal phototoxicity. The mouse respiratory system in inflammation models is a good example, as two-photon intravital imaging can provide insights as to how the immune system is activated in response to inflammation within the respiratory system. Inflammation models can be generated via influenza viral, bacterial, or lipopolysaccharide injection. To exteriorize the lungs or trachea, thoracotomy or tracheotomy is performed, respectively; the appropriate combination of inflammation induction and organ exposure is selected depending on the study purpose. On the other hand, visualizing the movement of leukocytes is also an important component; to this end, immune cell populations of interest are either labeled via the genetic attachment of fluorescent proteins or stained with antibodies or dyes. With the proper selection of methods at each step, two-photon intravital imaging can yield visual evidence regarding immune responses to inflammation.
MeSH Terms
Figure
Reference
-
1. Halin C, Mora JR, Sumen C, von Andrian UH. In vivo imaging of lymphocyte trafficking. Annu Rev Cell Dev Biol. 2005; 21:581–603.
Article2. Beerling E, Ritsma L, Vrisekoop N, Derksen PW, van Rheenen J. Intravital microscopy: new insights into metastasis of tumors. J Cell Sci. 2011; 124(Pt 3):299–310.
Article3. Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005; 2:932–40.
Article4. König K. Multiphoton microscopy in life sciences. J Microsc. 2000; 200(Pt 2):83–104.
Article5. Honda T, Otsuka A, Kabashima K. Novel insights into cutaneous immune systems revealed by in vivo imaging. Allergol Int. 2016; 65:228–34.6. Fiole D, Tournier JN. Intravital microscopy of the lung: minimizing invasiveness. J Biophotonics. 2016; 9:868–78.
Article7. Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, et al. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol. 2011; 300:F721–33.
Article8. Coppieters K, Martinic MM, Kiosses WB, Amirian N, von Herrath M. A novel technique for the in vivo imaging of autoimmune diabetes development in the pancreas by two-photon microscopy. PLoS One. 2010; 5:e15732.
Article9. Liang X, Grice JE, Zhu Y, Liu D, Sanchez WY, Li Z, et al. Intravital multiphoton imaging of the selective uptake of waterdispersible quantum dots into sinusoidal liver cells. Small. 2015; 11:1711–20.
Article10. Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science. 2015; 349:aaa4352.
Article11. Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017; 7:39.
Article12. Kukavica-Ibrulj I, Bragonzi A, Paroni M, Winstanley C, Sanschagrin F, O’Toole GA, et al. In vivo growth of Pseudomonas aeruginosa strains PAO1 and PA14 and the hypervirulent strain LESB58 in a rat model of chronic lung infection. J Bacteriol. 2008; 190:2804–13.13. Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev. 2005; 18:521–40.14. Lavoie EG, Wangdi T, Kazmierczak BI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect. 2011; 13:1133–45.
Article15. Lefrançais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2008; 3.
Article16. Looney MR, Bhattacharya J. Live imaging of the lung. Annu Rev Physiol. 2014; 76:431–45.
Article17. Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000; 406:782–7.
Article18. Opal SM. Endotoxins and other sepsis triggers. Contrib Nephrol. 2010; 167:14–24.
Article19. Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003; 3:169–76.
Article20. Osier M, Oberdörster G. Intratracheal inhalation vs intratracheal instillation: differences in particle effects. Fundam Appl Toxicol. 1997; 40:220–7.
Article21. Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF. Stabilized imaging of immune surveillance in the mouse lung. Nat Methods. 2011; 8:91–6.
Article22. Das S, MacDonald K, Chang HY, Mitzner W. A simple method of mouse lung intubation. J Vis Exp. 2013; (73):e50318.
Article23. Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000; 96:719–26.
Article24. Orthgiess J, Gericke M, Immig K, Schulz A, Hirrlinger J, Bechmann I, et al. Neurons exhibit Lyz2 promoter activity in vivo: implications for using LysM-Cre mice in myeloid cell research. Eur J Immunol. 2016; 46:1529–32.25. Garcia JA, Cardona SM, Cardona AE. Analyses of microglia effector function using CX3CR1-GFP knock-in mice. Methods Mol Biol. 2013; 1041:307–17.
Article26. Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011; 121:4787–95.
Article27. Noda S, Asano Y, Nishimura S, Taniguchi T, Fujiu K, Manabe I, et al. Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat Commun. 2014; 5:5797.
Article28. Park SA, Jeong S, Choe YH, Hyun YM. Sensing of vascular permeability in inflamed vessel of live animal. J Anal Methods Chem. 2018; 2018:5797152.
Article29. Lee PY, Wang JX, Parisini E, Dascher CC, Nigrovic PA. Ly6 family proteins in neutrophil biology. J Leukoc Biol. 2013; 94:585–94.
Article30. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013; 496:445–55.
Article31. Chiang EY, Hidalgo A, Chang J, Frenette PS. Imaging receptor microdomains on leukocyte subsets in live mice. Nat Methods. 2007; 4:219–22.
Article32. Gonçalves JT, Anstey JE, Golshani P, Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci. 2013; 16:903–9.
Article33. Issa JB, Haeffele BD, Agarwal A, Bergles DE, Young ED, Yue DT. Multiscale optical Ca2+ imaging of tonal organization in mouse auditory cortex. Neuron. 2014; 83:944–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neutrophil Extravasation Cascade: What Can We Learn from Two-photon Intravital Imaging?
- Real-time observation of neutrophil extracellular trap formation in the inflamed mouse brain via two-photon intravital imaging
- Intravital Laser-scanning Two-photon and Confocal Microscopy for Biomedical Research
- Intravital Two-photon Imaging of Dynamic Alteration of Hepatic Lipid Droplets in Fasted and Refed State
- Leucocyte Chemotaxis with Korean Ginseng