Int J Stem Cells.
2016 Nov;9(2):264-270. 10.15283/ijsc16052.
In vivo Evaluation of Human Embryonic Stem Cells Isolated by 57-C11 Monoclonal Antibody
- Affiliations
-
- 1Institute of Anticancer Medicine Development, Department of Integrative Bioscience and Biotechnology, Sejong University, Seoul, Korea. semidivine@hanmail.net cjryu@sejong.ac.kr
- KMID: 2362520
- DOI: http://doi.org/10.15283/ijsc16052
Abstract
- BACKGROUND
The normal cells derived from human embryonic stem cells (hESCs) are regarded as substitutes for damaged or dysfunctional adult cells. However, tumorigenicity of hESCs remains a major challenge in clinical application of hESC-derived cell transplantation. Previously, we generated monoclonal antibody (MAb) 57-C11 specific to the surface molecule on undifferentiated hESCs. The aim of this study is to prove whether 57-C11-positive hESCs are pluripotent and tumorigenic in immunodeficient mice.
METHODS
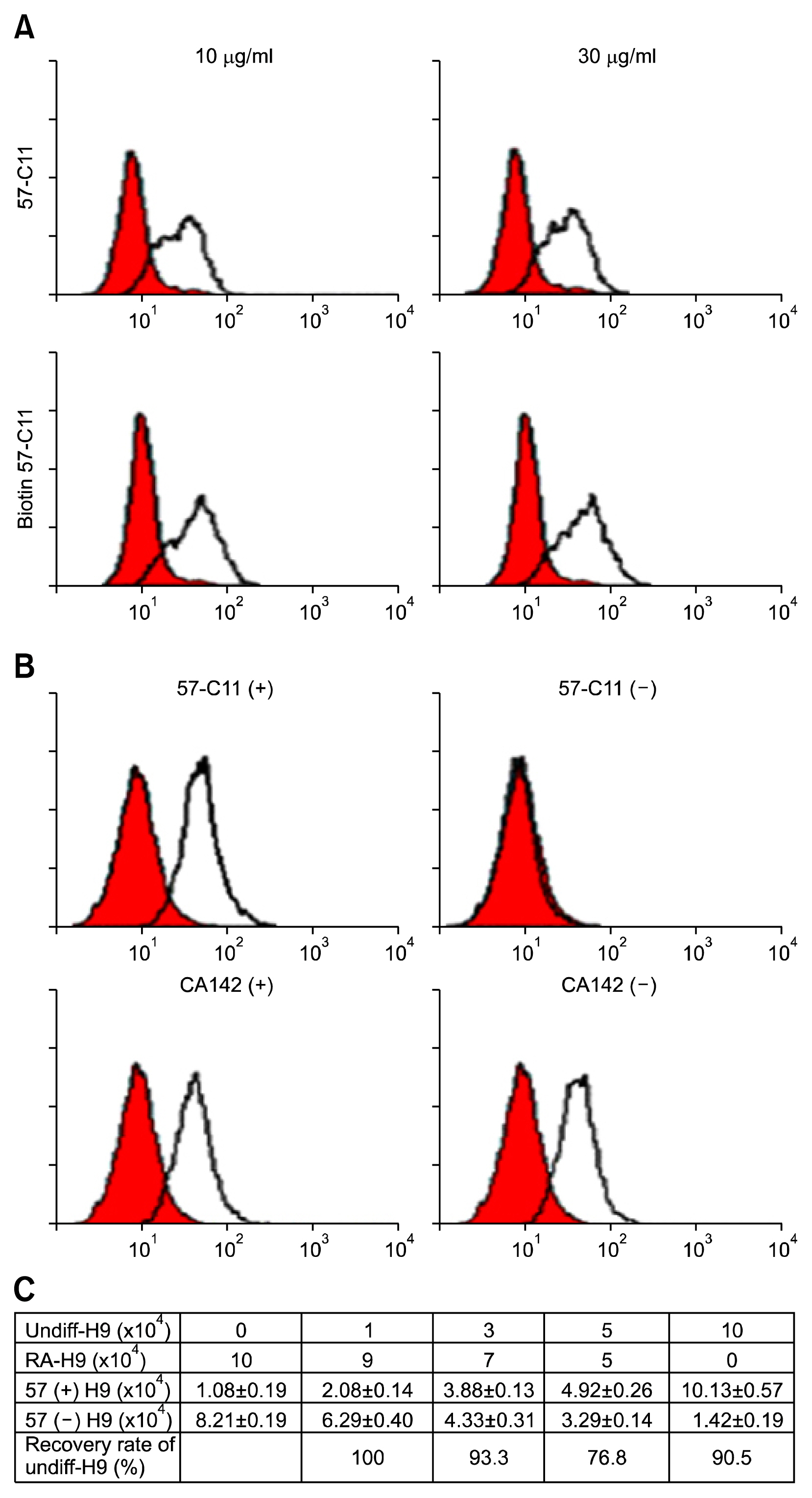
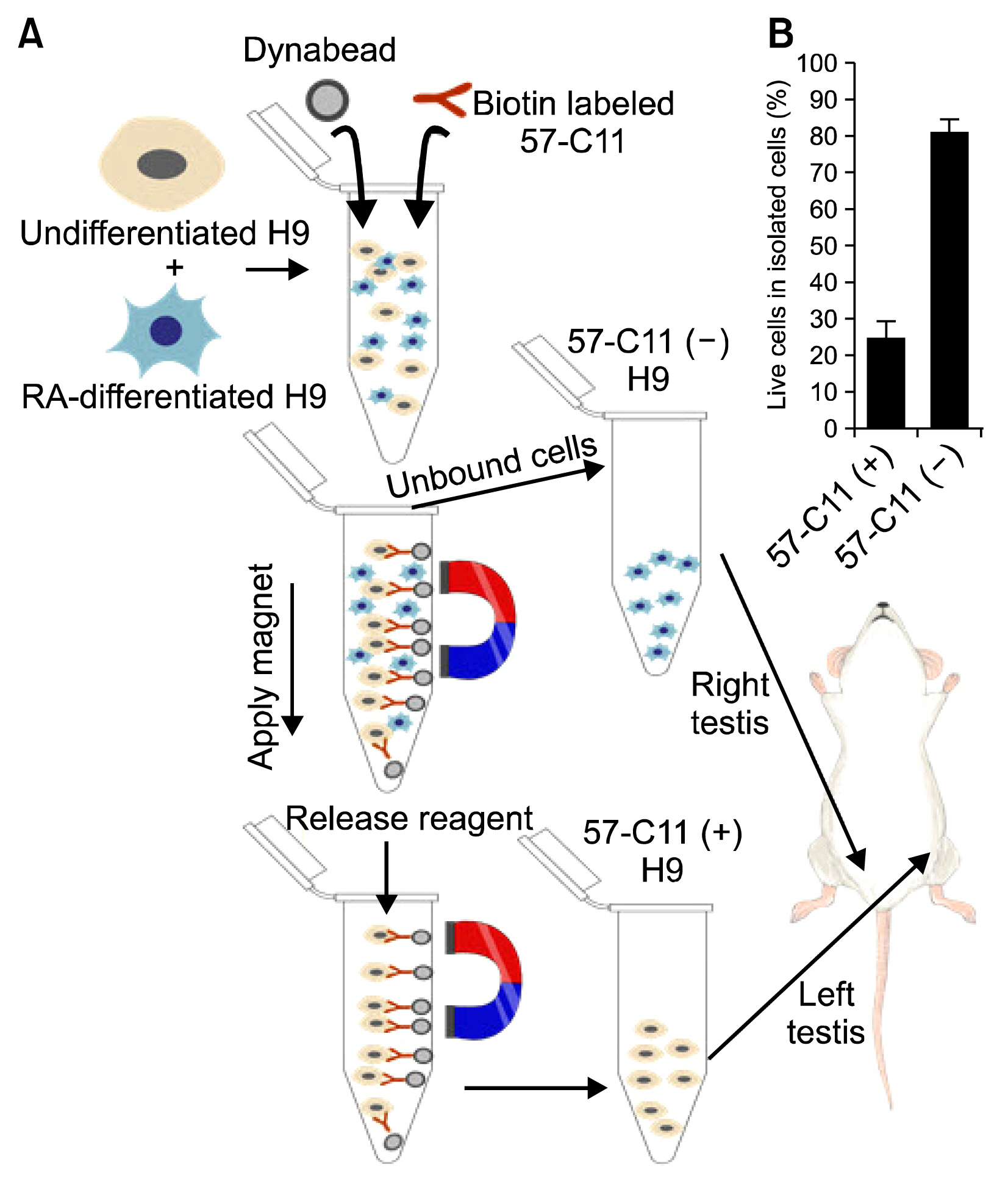
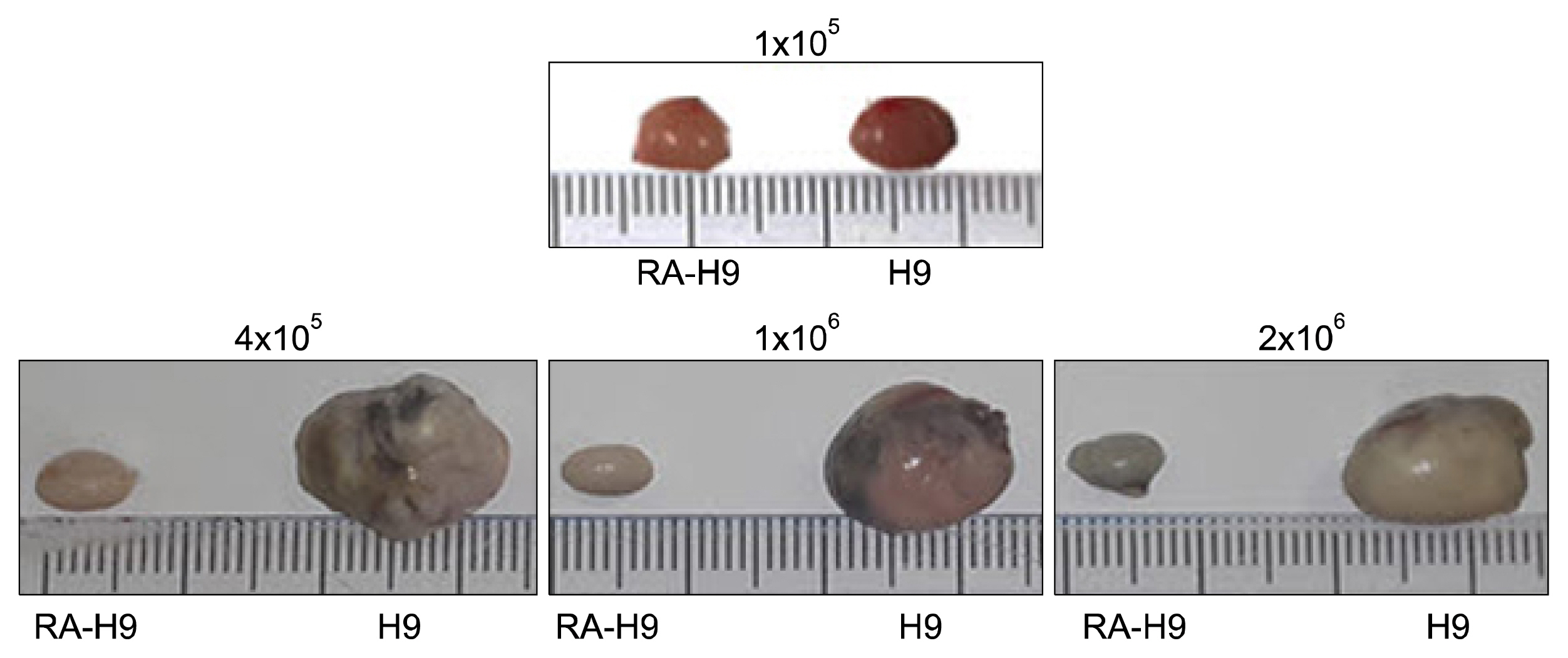
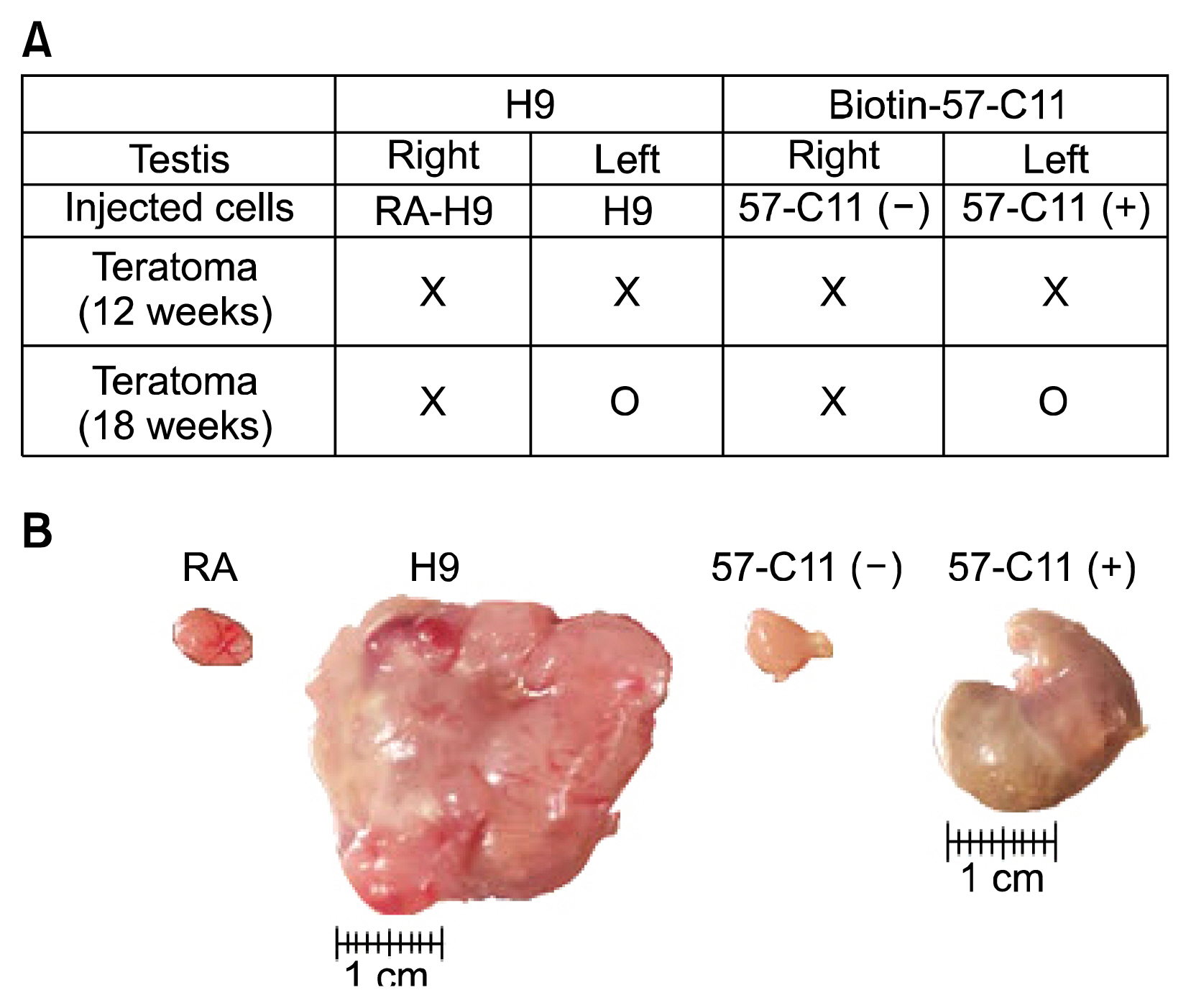
Undifferentiated hESCs were mixed with retinoic acid (RA)-differentiated hESCs at different ratios prior to 57-C11-mediated separation. To isolate 57-C11-positive hESCs from the mixture, biotinylated 57-C11 and streptavidin-coated magnetic beads were added to the mixture. Unbound 57-C11-negative hESCs were first isolated after applying magnet to the cell mixture, and 57-C11-bound hESCs were then released from the magnetic beads. In order to measure the efficiency of separation, 57-C11-positive or -negative hESCs were counted after isolation. To evaluate the efficiency of teratoma formation in vivo, 57-C11-positive or negative cells were further injected into left and right, respectively, testes of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice.
RESULTS
Approximately 77~100% of undifferentiated hESCs were isolated after applying 57-C11-coated magnetic beads to the mixed cell populations. Importantly, teratomas were not observed in NOD/SCID mice after the injection of isolated 57-C11-negative hESCs, whereas teratomas were observed with 57-C11-positive hESCs.
CONCLUSION
57-C11-positive hESCs are pluripotent and tumorigenic. The combination of 57-C11 and magnetic beads will be useful to eliminate remaining undifferentiated hESCs for the safe cell transplantation.
MeSH Terms
Figure
Reference
-
References
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.
Article2. Vogel G. Cell biology. Ready or not? Human ES cells head toward the clinic. Science. 2005; 308:1534–1538. DOI: 10.1126/science.308.5728.1534. PMID: 15947149.3. Hentze H, Graichen R, Colman A. Cell therapy and the safety of embryonic stem cell-derived grafts. Trends Biotechnol. 2007; 25:24–32. DOI: 10.1016/j.tibtech.2006.10.010.
Article4. Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001; 19:1129–1133. DOI: 10.1038/nbt1201-1129. PMID: 11731781.
Article5. Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005; 167:663–671. DOI: 10.1016/S0002-9440(10)62041-X. PMID: 16127147. PMCID: 1698736.
Article6. Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006; 24:1433–1440. DOI: 10.1634/stemcells.2005-0393. PMID: 16556709.
Article7. Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006; 12:1259–1268. DOI: 10.1038/nm1495. PMID: 17057709.
Article8. Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, Yoshikawa T, Hayashi H, Shinoyama M, Refaat MM, Suemori H, Miyamoto S, Takahashi J. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells. 2012; 30:935–945. DOI: 10.1002/stem.1060. PMID: 22328536.
Article9. Seminatore C, Polentes J, Ellman D, Kozubenko N, Itier V, Tine S, Tritschler L, Brenot M, Guidou E, Blondeau J, Lhuillier M, Bugi A, Aubry L, Jendelova P, Sykova E, Perrier AL, Finsen B, Onteniente B. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke. 2010; 41:153–159. DOI: 10.1161/STROKEAHA.109.563015.
Article10. Choi HS, Kim H, Won A, Kim JJ, Son CY, Kim KS, Ko JH, Lee MY, Kim CH, Ryu CJ. Development of a decoy immunization strategy to identify cell-surface molecules expressed on undifferentiated human embryonic stem cells. Cell Tissue Res. 2008; 333:197–206. DOI: 10.1007/s00441-008-0632-6. PMID: 18560898.
Article11. Choi HS, Kim WT, Kim H, Kim JJ, Ko JY, Lee SW, Jang YJ, Kim SJ, Lee MJ, Jung HS, Kzhyshkowska J, Um SJ, Lee MY, Lee SH, Kim CH, Ryu CJ. Identification and characterization of adenovirus early region 1B-associated protein 5 as a surface marker on undifferentiated human embryonic stem cells. Stem Cells Dev. 2011; 20:609–620. DOI: 10.1089/scd.2010.0265.
Article12. Gabler S, Schütt H, Groitl P, Wolf H, Shenk T, Dobner T. E1B 55-kilodalton-associated protein: a cellular protein with RNA-binding activity implicated in nucleocytoplasmic transport of adenovirus and cellular mRNAs. J Virol. 1998; 72:7960–7971. PMID: 9733834. PMCID: 110131.
Article13. Kim WT, Seo Choi H, Min Lee H, Jang YJ, Ryu CJ. B-cell receptor-associated protein 31 regulates human embryonic stem cell adhesion, stemness, and survival via control of epithelial cell adhesion molecule. Stem Cells. 2014; 32:2626–2641. DOI: 10.1002/stem.1765. PMID: 24898727.
Article14. Kim WT, Choi HS, Hwang HJ, Jung HS, Ryu CJ. Epitope mapping of antibodies suggests the novel membrane topology of B-cell receptor associated protein 31 on the cell surface of embryonic stem cells: the novel membrane topology of BAP31. PLoS One. 2015; 10:e0130670. DOI: 10.1371/journal.pone.0130670. PMID: 26102500. PMCID: 4478030.
Article15. Choi HS, Lee HM, Jang YJ, Kim CH, Ryu CJ. Heterogeneous nuclear ribonucleoprotein A2/B1 regulates the self-renewal and pluripotency of human embryonic stem cells via the control of the G1/S transition. Stem Cells. 2013; 31:2647–2658. DOI: 10.1002/stem.1366. PMID: 23495120.
Article16. Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009; 2:198–210. DOI: 10.1016/j.scr.2009.02.002. PMID: 19393593.
Article17. Choi HS, Lee HM, Kim WT, Kim MK, Chang HJ, Lee HR, Joh JW, Kim DS, Ryu CJ. Detection of mycoplasma infection in circulating tumor cells in patients with hepatocellular carcinoma. Biochem Biophys Res Commun. 2014; 446:620–625. DOI: 10.1016/j.bbrc.2014.03.024. PMID: 24637212.
Article18. Choo AB, Tan HL, Ang SN, Fong WJ, Chin A, Lo J, Zheng L, Hentze H, Philp RJ, Oh SK, Yap M. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells. 2008; 26:1454–1463. DOI: 10.1634/stemcells.2007-0576. PMID: 18356574.
Article19. Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007; 25:681–686. DOI: 10.1038/nbt1310. PMID: 17529971.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Concepts of Stem Cell Therapy
- Problems Associated with Establishment of Human Embryonic Stem Cell
- Stem Cells in Drug Screening for Neurodegenerative Disease
- Assessment of Developmental Toxicants using Human Embryonic Stem Cells
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy