Clin Exp Otorhinolaryngol.
2016 Dec;9(4):374-381. 10.21053/ceo.2015.01683.
Parameters of Stromal Activation and Epithelial to Mesenchymal Transition as Predictive Biomarkers for Induction Chemotherapy in Patients With Locally Advanced Oral Cavity and Oropharyngeal Squamous Cell Cancer
- Affiliations
-
- 1Institute of Pathology, Jena University Hospital, Jena, Germany.
- 2Department of Otorhinolaryngology, Jena University Hospital, Jena, Germany. orlando.guntinas@med.uni-jena.de
- KMID: 2360771
- DOI: http://doi.org/10.21053/ceo.2015.01683
Abstract
OBJECTIVES
Induction chemotherapy (IC) is likely to be effective for biologically distinct subgroups of oral cancer and biomarker development may lead to identification of those patients.
METHODS
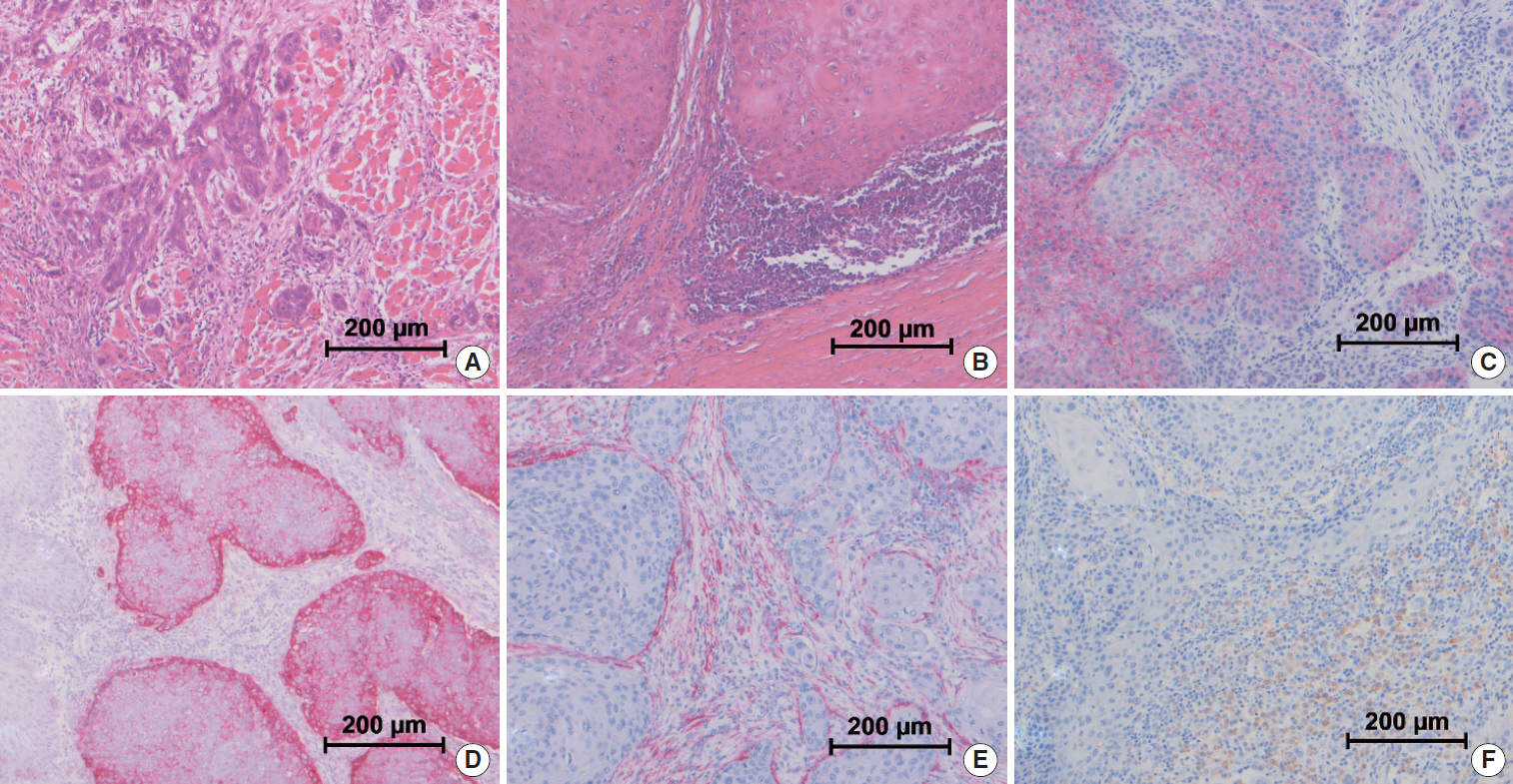
We evaluated immune cell infiltration, stroma formation and structure of the invasive front as well as the immunohistochemical expression of alpha smooth muscle actin (ASMA), CD163, E-cadherin, N-cadherin, and the laminin gamma 2 chain in pretreatment biopsy specimens and surgical resections after IC in 20 patients with locally advanced oral cancer who were treated in a prospective, ongoing, phase II trial on IC using docetaxel, cisplatin, and 5-fluorouracil (TPF).
RESULTS
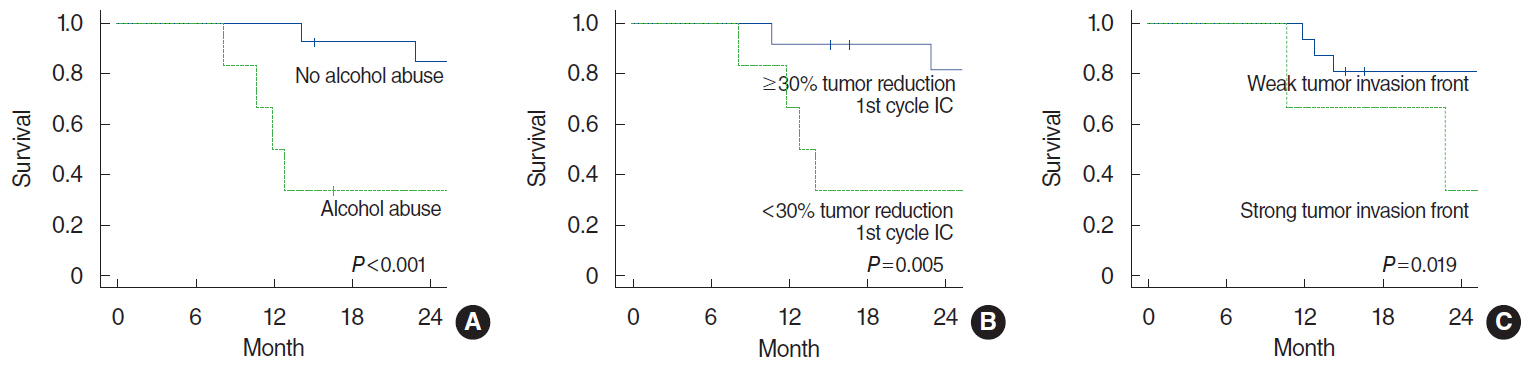
Significant negative prognostic factors for incomplete pathological tumor response to IC were alcohol abuse (P=0.032), cN+ (P=0.042), and <30% tumor reduction after first cycle of IC (P=0.034). Of the investigated histological parameters and biomarkers only a low membrane-bound expression of E-cadherin showed a trend to be associated with incomplete response to IC (P=0.061). Low expression of ASMA in stromal vessels and a strong tumor invasion front were significantly associated to tumor recurrence (P=0.024 and P=0.004, respectively). The median follow-up of all patients was 35 months. Alcohol abuse (P<0.001), <30% tumor reduction after first cycle of IC (P=0.005), and a strong tumor invasion front (P=0.019) were negative prognostic factors for overall survival.
CONCLUSION
A strong predictive biomarker among the investigated parameters for benefitting from TPF IC could not be found. The extent of the tumor invasion front was a negative prognostic marker for recurrence and survival in oral cancer treated by TPF IC followed by surgery and postoperative radiochemotherapy.
MeSH Terms
-
Actins
Alcoholism
Biomarkers*
Biopsy
Cadherins
Chemoradiotherapy
Cisplatin
Epithelial Cells*
Fluorouracil
Follow-Up Studies
Humans
Induction Chemotherapy*
Laminin
Mouth Neoplasms
Mouth*
Muscle, Smooth
Neoplasm Invasiveness
Neoplasms, Squamous Cell*
Prospective Studies
Recurrence
Actins
Biomarkers
Cadherins
Cisplatin
Fluorouracil
Laminin
Figure
Reference
-
1. Argiris A. Current status and future directions in induction chemotherapy for head and neck cancer. Crit Rev Oncol Hematol. 2013; Oct. 88(1):57–74.
Article2. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007; Oct. 357(17):1705–15.
Article3. Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007; Oct. 357(17):1695–704.
Article4. Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013; Mar. 14(3):257–64.
Article5. Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014; Sep. 32(25):2735–43.
Article6. Benasso M. Induction chemotherapy for squamous cell head and neck cancer: a neverending story? Oral Oncol. 2013; Aug. 49(8):747–52.
Article7. Hanna GJ, Haddad RI, Lorch JH. Induction chemotherapy for locoregionally advanced head and neck cancer: past, present, future? Oncologist. 2013; 18(3):288–93.
Article8. Yang CZ, Ma J, Zhu DW, Liu Y, Montgomery B, Wang LZ, et al. GDF15 is a potential predictive biomarker for TPF induction chemotherapy and promotes tumorigenesis and progression in oral squamous cell carcinoma. Ann Oncol. 2014; Jun. 25(6):1215–22.
Article9. Zhu DW, Liu Y, Yang X, Yang CZ, Ma J, Yang X, et al. Low Annexin A1 expression predicts benefit from induction chemotherapy in oral cancer patients with moderate or poor pathologic differentiation grade. BMC Cancer. 2013; Jun. 13:301.
Article10. Kim MJ, Ki MS, Kim K, Shim HJ, Hwang JE, Bae WK, et al. Different protein expression associated with chemotherapy response in oropharyngeal cancer according to HPV status. BMC Cancer. 2014; Nov. 14:824.
Article11. Pectasides E, Rampias T, Sasaki C, Perisanidis C, Kouloulias V, Burtness B, et al. Markers of epithelial to mesenchymal transition in association with survival in head and neck squamous cell carcinoma (HNSCC). PLoS One. 2014; Apr. 9(4):e94273.
Article12. Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013; Apr. 49(4):287–92.
Article13. Vig N, Mackenzie IC, Biddle A. Phenotypic plasticity and epithelial-to-mesenchymal transition in the behaviour and therapeutic response of oral squamous cell carcinoma. J Oral Pathol Med. 2015; Oct. 44(9):649–55.
Article14. Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010; Oct. 316(17):2713–22.15. Fujii N, Shomori K, Shiomi T, Nakabayashi M, Takeda C, Ryoke K, et al. Cancer-associated fibroblasts and CD163-positive macrophages in oral squamous cell carcinoma: their clinicopathological and prognostic significance. J Oral Pathol Med. 2012; Jul. 41(6):444–51.
Article16. Ding L, Zhang Z, Shang D, Cheng J, Yuan H, Wu Y, et al. α-Smooth muscle actin-positive myofibroblasts, in association with epithelial-mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J Oral Pathol Med. 2014; May. 43(5):335–43.
Article17. Zhou B, Chen WL, Wang YY, Lin ZY, Zhang DM, Fan S, et al. A role for cancer-associated fibroblasts in inducing the epithelial-to-mesenchymal transition in human tongue squamous cell carcinoma. J Oral Pathol Med. 2014; Sep. 43(8):585–92.
Article18. Oertel K, Spiegel K, Schmalenberg H, Dietz A, Maschmeyer G, Kuhnt T, et al. Phase I trial of split-dose induction docetaxel, cisplatin, and 5-fluorouracil (TPF) chemotherapy followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer (TISOC-1). BMC Cancer. 2012; Oct. 12:483.
Article19. Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987; Jun. 95(3):229–49.
Article20. Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989; Sep. 18(8):432–7.21. Cullen KJ, Schumaker L, Nikitakis N, Goloubeva O, Tan M, Sarlis NJ, et al. beta-Tubulin-II expression strongly predicts outcome in patients receiving induction chemotherapy for locally advanced squamous carcinoma of the head and neck: a companion analysis of the TAX 324 trial. J Clin Oncol. 2009; Dec. 27(36):6222–8.22. Wu Y, Posner MR, Schumaker LM, Nikitakis N, Goloubeva O, Tan M, et al. Novel biomarker panel predicts prognosis in human papillomavirus-negative oropharyngeal cancer: an analysis of the TAX 324 trial. Cancer. 2012; Apr. 118(7):1811–7.23. Jaiswal JK, Nylandsted J. S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells. Cell Cycle. 2015; 14(4):502–9.
Article24. Franz M, Richter P, Geyer C, Hansen T, Acuna LD, Hyckel P, et al. Mesenchymal cells contribute to the synthesis and deposition of the laminin-5 gamma2 chain in the invasive front of oral squamous cell carcinoma. J Mol Histol. 2007; Jun. 38(3):183–90.25. Franz M, Wolheim A, Richter P, Umbreit C, Dahse R, Driemel O, et al. Stromal laminin chain distribution in normal, hyperplastic and malignant oral mucosa: relation to myofibroblast occurrence and vessel formation. J Oral Pathol Med. 2010; Apr. 39(4):290–8.
Article26. He KF, Zhang L, Huang CF, Ma SR, Wang YF, Wang WM, et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed Res Int. 2014; 2014:838632.
Article27. Hanemann JA, Oliveira DT, Nonogaki S, Nishimoto IN, de Carli ML, Landman G, et al. Expression of E-cadherin and β-catenin in basaloid and conventional squamous cell carcinoma of the oral cavity: are potential prognostic markers? BMC Cancer. 2014; Jun. 14:395.
Article28. Walker A, Frei R, Lawson KR. The cytoplasmic domain of N-cadherin modulates MMP-9 induction in oral squamous carcinoma cells. Int J Oncol. 2014; Oct. 45(4):1699–706.
Article29. Martone T, Rosso P, Albera R, Migliaretti G, Fraire F, Pignataro L, et al. Prognostic relevance of CD105+ microvessel density in HNSCC patient outcome. Oral Oncol. 2005; Feb. 41(2):147–55.
Article30. Marioni G, Staffieri A, Fasanaro E, Stramare R, Giacomelli L, Bernardi E, et al. The role of angiogenin in pT1-T2 tongue carcinoma neo-angiogenesis and cell proliferation: an exploratory study. J Oral Pathol Med. 2013; Sep. 42(8):606–11.
Article31. Almangush A, Bello IO, Keski-Santti H, Makinen LK, Kauppila JH, Pukkila M, et al. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014; Jun. 36(6):811–8.
Article32. Szybiak B, Korski K, Golusinski W. Role of extended histological examination in the assessment of local recurrence of the oral cancer. Otolaryngol Pol. 2015; 69(1):17–21.
Article33. Kies MS, Holsinger FC, Lee JJ, William WN Jr, Glisson BS, Lin HY, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010; Jan. 28(1):8–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Organ Preservation for the Management of Locally Advanced Head and Neck Cancer
- Lysophosphatidic Acid-Induced TWIST1 and Slug Expression in Oral Cancer Cell Invasion
- The Epithelial-Mesenchymal Transition and E-cadherin and Vimentin Expression in Basal Cell Carcinoma and Squamous Cell Carcinoma
- The Prognostic Value of DNA Flow Cytometry in Patients with Early Primary Oral Cavity and Oropharyngeal Squamous Cell Carcinoma
- Tivozanib-induced activation of the mitochondrial apoptotic pathway and suppression of epithelial-to-mesenchymal transition in oral squamous cell carcinoma