J Korean Soc Radiol.
2016 Dec;75(6):419-433. 10.3348/jksr.2016.75.6.419.
Amide Proton Transfer Imaging in Clinics: Basic Concepts and Current and Future Use in Brain Tumors and Stroke
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 2Department of Radiology, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, Korea.
- 3Philips Korea, Seoul, Korea. hakyu.jeong@gmail.com
- KMID: 2360429
- DOI: http://doi.org/10.3348/jksr.2016.75.6.419
Abstract
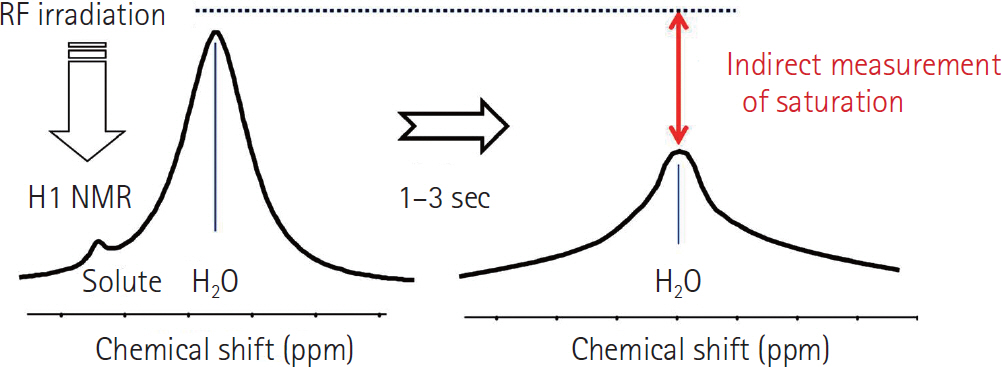
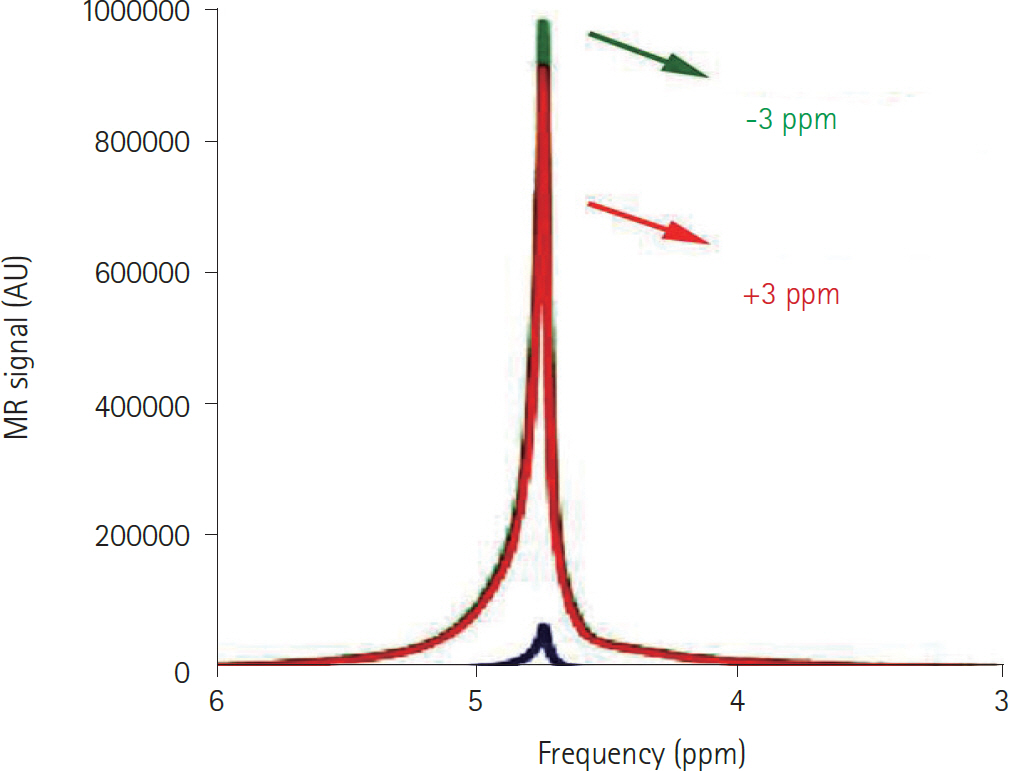
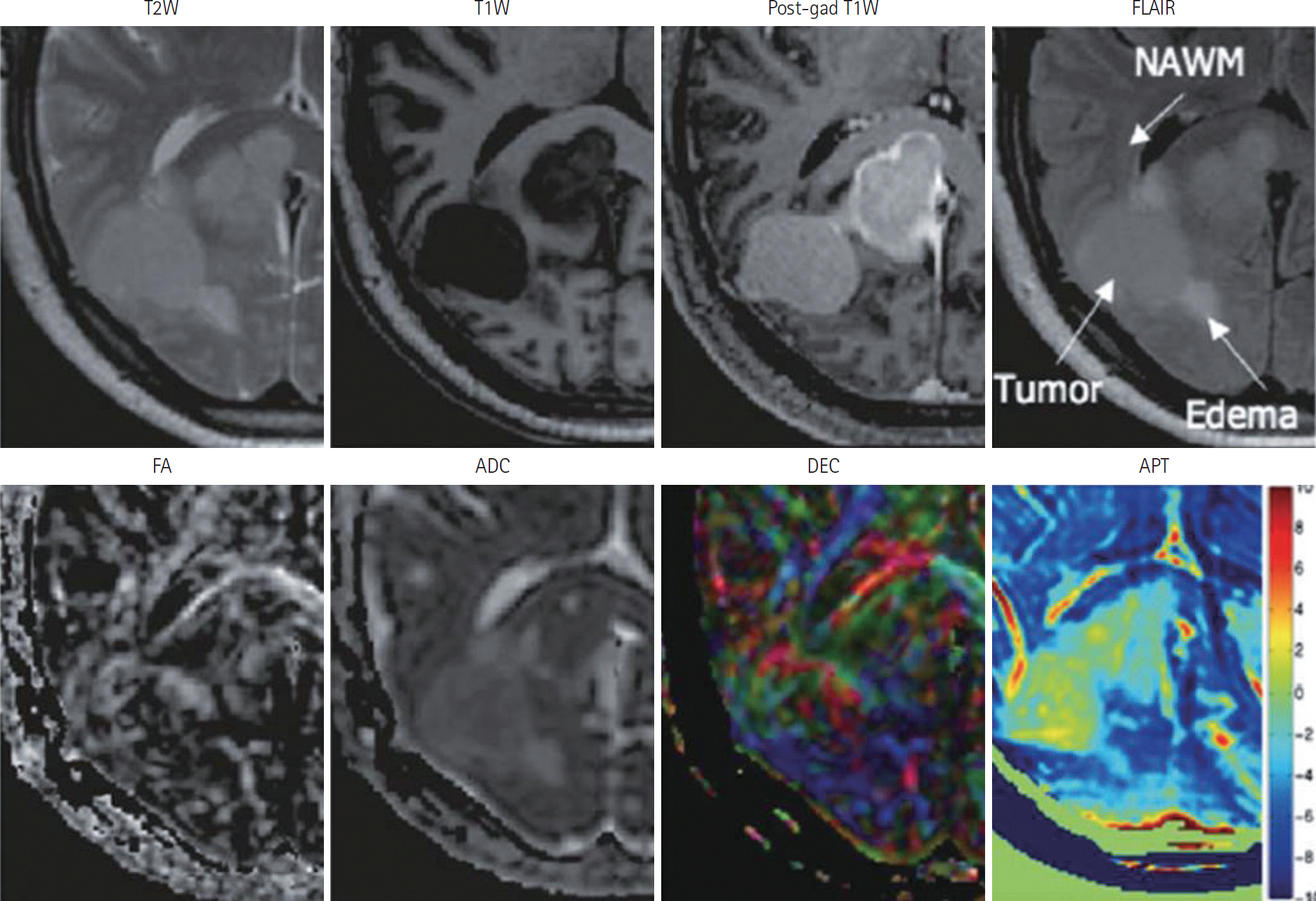
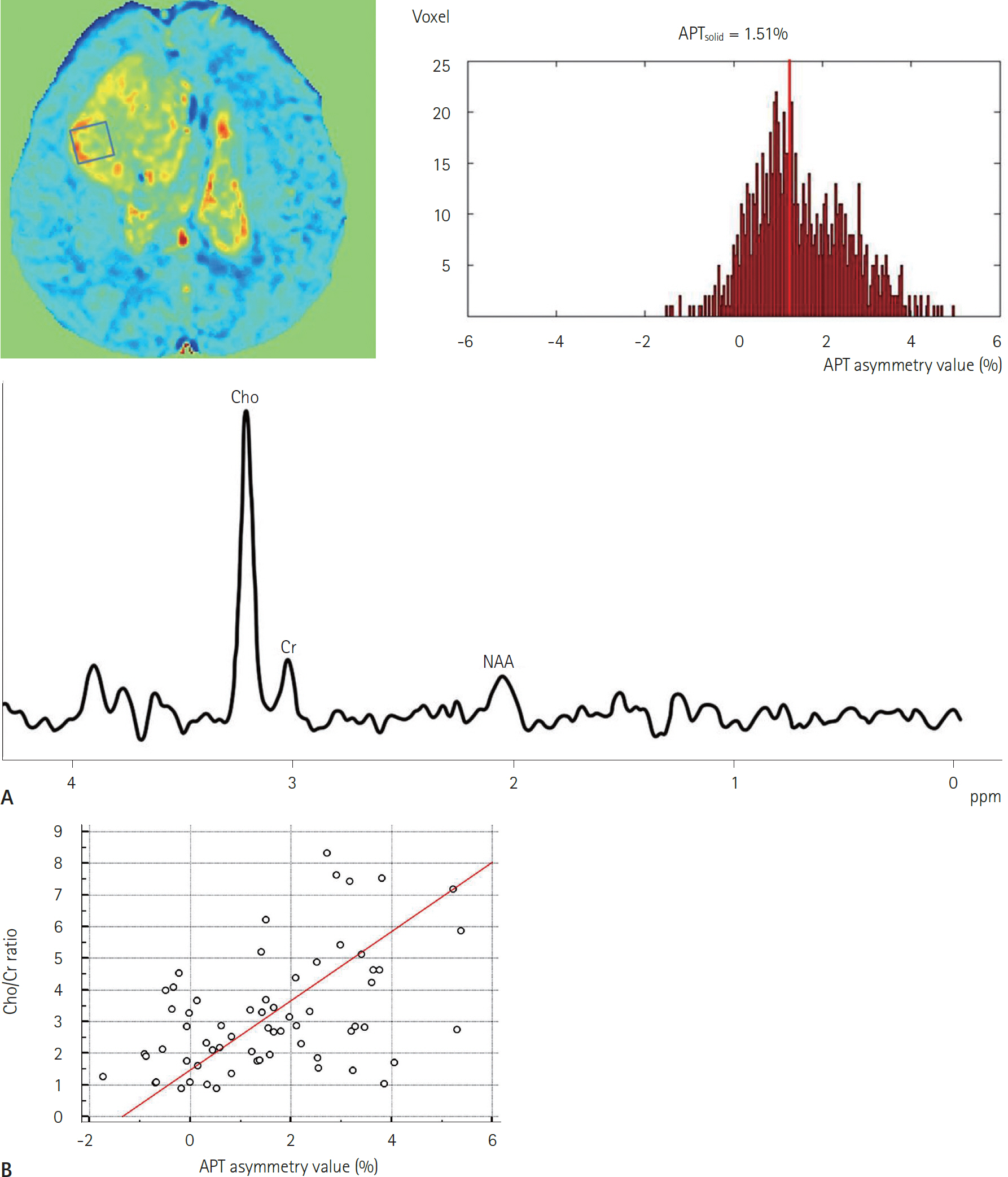
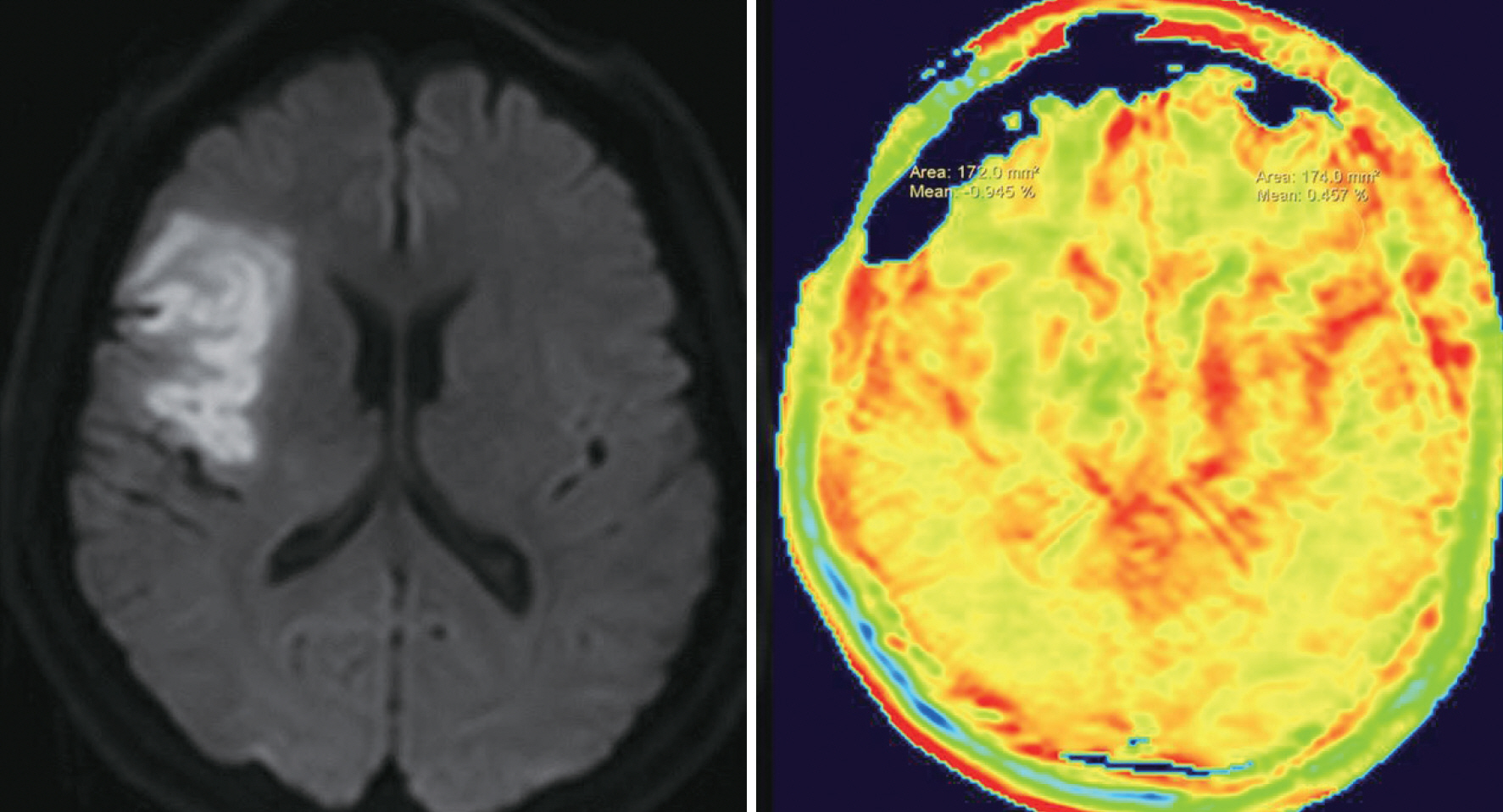
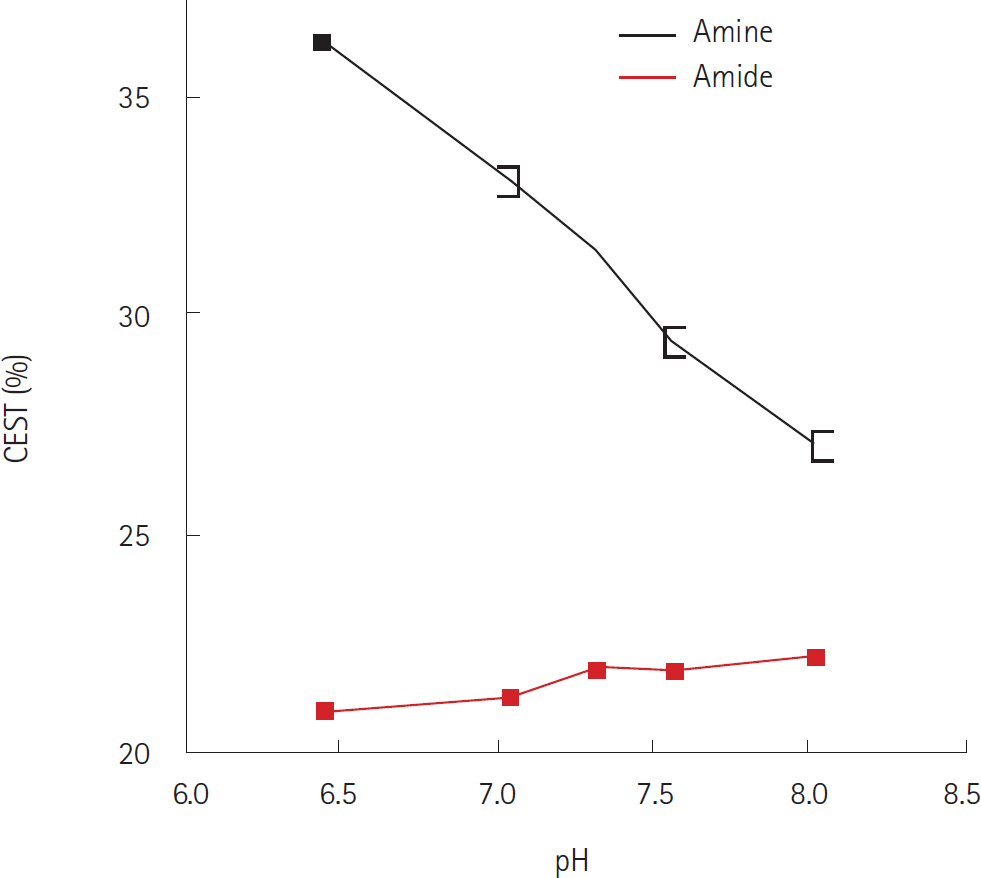
- Amide proton transfer (APT) imaging is gaining attention as a relatively new in vivo molecular imaging technique that has higher sensitivity and spatial resolution than magnetic resonance spectroscopy imaging. APT imaging is a subset of the chemical exchange saturation transfer mechanism, which can offer unique image contrast by selectively saturating protons in target molecules that get exchanged with protons in bulk water. In this review, we describe the basic concepts of APT imaging, particularly with regard to the benefit in clinics from the current literature. Clinical applications of APT imaging are described from two perspectives: in the diagnosis and monitoring of the treatment response in brain glioma by reflecting endogenous mobile proteins and peptides, and in the potential for stroke imaging with respect to tissue acidity.
MeSH Terms
Figure
Cited by 1 articles
-
Magnetic Resonance Imaging: Historical Overview, Technical Developments, and Clinical Applications
Geon-Ho Jahng, Soonchan Park, Chang-Woo Ryu, Zang-Hee Cho
Prog Med Phys. 2020;31(3):35-53. doi: 10.14316/pmp.2020.31.3.35.
Reference
-
1. Zaiss M, Bachert P. Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: a review of theo-retical approaches and methods. Phys Med Biol. 2013; 58:R221–R269.2. van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med. 2011; 65:927–948.
Article3. Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, et al. Magnetic resonance imaging of glutamate. Nat Med. 2012; 18:302–306.
Article4. Sun PZ, van Zijl PC, Zhou J. Optimization of the irradiation power in chemical exchange dependent saturation transfer experiments. J Magn Reson. 2005; 175:193–200.
Article5. Baguet E, Roby C. Off-resonance irradiation effect in steady-state NMR saturation transfer. J Magn Reson. 1997; 128:149–160.
Article6. Baguet E, Roby C. Fast inversion-recovery measurements in the presence of a saturating field for a 2-spin system in chemical-exchange. J Magn Reson Ser A. 1994; 108:189–195.7. Scheidegger R, Wong ET, Alsop DC. Contributors to contrast between glioma and brain tissue in chemical exchange saturation transfer sensitive imaging at 3 Tesla. Neuroimage. 2014; 99:256–268.8. Zong X, Wang P, Kim SG, Jin T. Sensitivity and source of amine-proton exchange and amide-proton transfer magnetic resonance imaging in cerebral ischemia. Magn Reson Med. 2014; 71:118–132.
Article9. Lee DH, Heo HY, Zhang K, Zhang Y, Jiang S, Zhao X, et al. Quantitative assessment of the effects of water proton concentration and water T1 changes on amide proton transfer (APT) and nuclear overhauser enhancement (NOE) MRI: The origin of the APT imaging signal in brain tumor. Magn Reson Med 2016 Feb 2 [Epub].http://dx.doi.org/10.1002/mrm.26131.10. Xu J, Zaiss M, Zu Z, Li H, Xie J, Gochberg DF, et al. On the origins of chemical exchange saturation transfer (CEST) contrast in tumors at 9.4 T. NMR Biomed. 2014; 27:406–416.11. Zaiss M, Windschuh J, Goerke S, Paech D, Meissner JE, Burth S, et al. Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn Reson Med 2016 Jan 27 [Epub].http://dx.doi.org/10.1002/mrm.26100.12. Zhu H, Jones CK, van Zijl PC, Barker PB, Zhou J. Fast 3D chemical exchange saturation transfer (CEST) imaging of the human brain. Magn Reson Med. 2010; 64:638–644.
Article13. Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009; 61:1441–1450.
Article14. Zhou JY, van Zijl PCM. Chemical exchange saturation transfer imaging and spectroscopy. Prog Nucl Magn Reson Spectrosc. 2006; 48:109–136.
Article15. Sun PZ, Farrar CT, Sorensen AG. Correction for artifacts induced by B(0) and B(1) field inhomogeneities in pH-sen-sitive chemical exchange saturation transfer (CEST) imaging. Magn Reson Med. 2007; 58:1207–1215.16. Zhao X, Wen Z, Zhang G, Huang F, Lu S, Wang X, et al. Three-dimensional turbo-spin-echo amide proton transfer MR imaging at 3-Tesla and its application to high-grade human brain tumors. Mol Imaging Biol. 2013; 15:114–122.
Article17. Zhou J, Wilson DA, Sun PZ, Klaus JA, Van Zijl PC. Quantitative description of proton exchange processes between water and endogenous and exogenous agents for WEX, CEST, and APT experiments. Magn Reson Med. 2004; 51:945–952.
Article18. Zhou J, Zhu H, Lim M, Blair L, Quinones-Hinojosa A, Mes-sina SA, et al. Three-dimensional amide proton transfer MR imaging of gliomas: initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging. 2013; 38:1119–1128.
Article19. Zhou J. Amide proton transfer imaging of the human brain. Methods Mol Biol. 2011; 711:227–237.
Article20. Deng M, Chen SZ, Yuan J, Chan Q, Zhou J, Wáng YX. Chemical exchange saturation transfer (CEST) MR technique for liver imaging at 3.0 Tesla: an evaluation of different offset number and an after-meal and over-night-fast comparison. Mol Imaging Biol. 2016; 18:274–282.
Article21. Keupp J, Eggers H. CEST-dixon MRI for sensitive and accu-rate measurement of amide proton transfer in humans at 3T. Proc Intl Soc Mag Reson Med. 2010; 18:338.22. Tietze A, Blicher J, Mikkelsen IK, Østergaard L, Strother MK, Smith SA, et al. Assessment of ischemic penumbra in patients with hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI. NMR Biomed. 2014; 27:163–174.
Article23. Sled JG, Pike GB. Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magn Reson Med. 2001; 46:923–931.
Article24. Smith SA, Farrell JA, Jones CK, Reich DS, Calabresi PA, van Zijl PC. Pulsed magnetization transfer imaging with body coil transmission at 3 Tesla: feasibility and application. Magn Reson Med. 2006; 56:866–875.
Article25. Jones CK, Huang A, Xu J, Edden RA, Schär M, Hua J, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. Neuroimage. 2013; 77:114–124.
Article26. Zhou J, Blakeley JO, Hua J, Kim M, Laterra J, Pomper MG, et al. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med. 2008; 60:842–849.
Article27. Jones CK, Schlosser MJ, van Zijl PC, Pomper MG, Golay X, Zhou J. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006; 56:585–592.
Article28. Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Us-ing the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003; 9:1085–1090.
Article29. Hua J, Hurst GC. Analysis of on- and off-resonance magnetization transfer techniques. J Magn Reson Imaging. 1995; 5:113–120.
Article30. Desmond KL, Moosvi F, Stanisz GJ. Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7 T. Magn Reson Med. 2014; 71:1841–1853.
Article31. Meissner JE, Windschuh J, Zaiss M, Paech D, Radbruch A, Bachert P. Multi-pool CEST imaging of glioblastoma at 7 T. Milano: Proceedings of the 22nd Annual Meeting of ISMRM,. 2014. 3152.32. Zaiss M, Schmitt B, Bachert P. Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. J Magn Reson. 2011; 211:149–155.33. Woessner DE, Zhang S, Merritt ME, Sherry AD. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med. 2005; 53:790–799.
Article34. Jin T, Wang P, Zong X, Kim SG. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med. 2013; 69:760–770.
Article35. Jeong HK, Smith S, Kim HS. Usefulness of three-pool lo-rentzian model in estimating APT efect and parameteriz-ing spectral curve shape. Milano: Proceedings of the 22nd Annual Meeting of ISMRM,. 2014. 3305.36. Stancanello J, Terreno E, Castelli DD, Cabella C, Uggeri F, Aime S. Development and validation of a smoothing-splines-based correction method for improving the analysis of CEST-MR images. Contrast Media Mol Imaging. 2008; 3:136–149.
Article37. Moon BF, Jones KM, Chen LQ, Liu P, Randtke EA, Howison CM, et al. A comparison of iopromide and iopamidol, two acidoCEST MRI contrast media that measure tumor extra-cellular pH. Contrast Media Mol Imaging. 2015; 10:446–455.
Article38. McVicar N, Li AX, Gonçalves DF, Bellyou M, Meakin SO, Prado MA, et al. Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentra-tion-independent detection (AACID) with MRI. J Cereb Blood Flow Metab. 2014; 34:690–698.
Article39. Wu R, Longo DL, Aime S, Sun PZ. Quantitative description of radiofrequency (RF) power-based ratiometric chemical exchange saturation transfer (CEST) pH imaging. NMR Biomed. 2015; 28:555–565.
Article40. Ward KM, Balaban RS. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST). Magn Reson Med. 2000; 44:799–802.
Article41. Müller-Lutz A, Khalil N, Schmitt B, Jellus V, Pentang G, Oeltzschner G, et al. Pilot study of Iopamidol-based quan-titative pH imaging on a clinical 3T MR scanner. MAGMA. 2014; 27:477–485.
Article42. Longo DL, Dastrù W, Digilio G, Keupp J, Langereis S, Lan-zardo S, et al. Iopamidol as a responsive MRI-chemical exchange saturation transfer contrast agent for pH mapping of kidneys: in vivo studies in mice at 7 T. Magn Reson Med. 2011; 65:202–211.
Article43. Zu Z, Janve VA, Li K, Does MD, Gore JC, Gochberg DF. Multi-angle ratiometric approach to measure chemical exchange in amide proton transfer imaging. Magn Reson Med. 2012; 68:711–719.
Article44. Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003; 50:1120–1126.
Article45. Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991; 64:425–427.
Article46. Togao O, Yoshiura T, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol. 2014; 16:441–448.
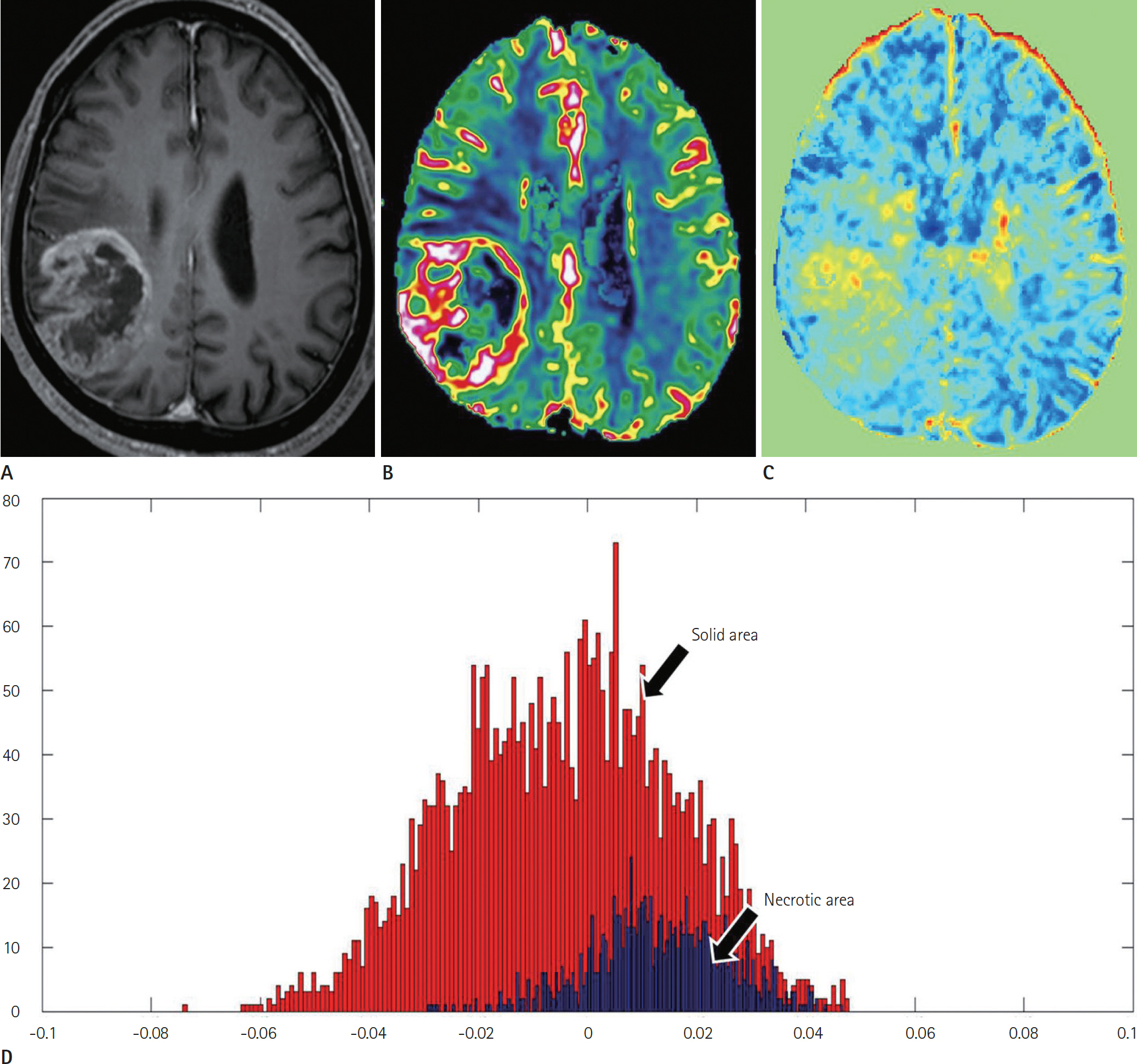
Article47. Park JE, Kim HS, Park KJ, Kim SJ, Kim JH, Smith SA. Pre- and posttreatment glioma: comparison of amide proton transfer imaging with MR spectroscopy for biomarkers of tumor proliferation. Radiology. 2016; 278:514–523.
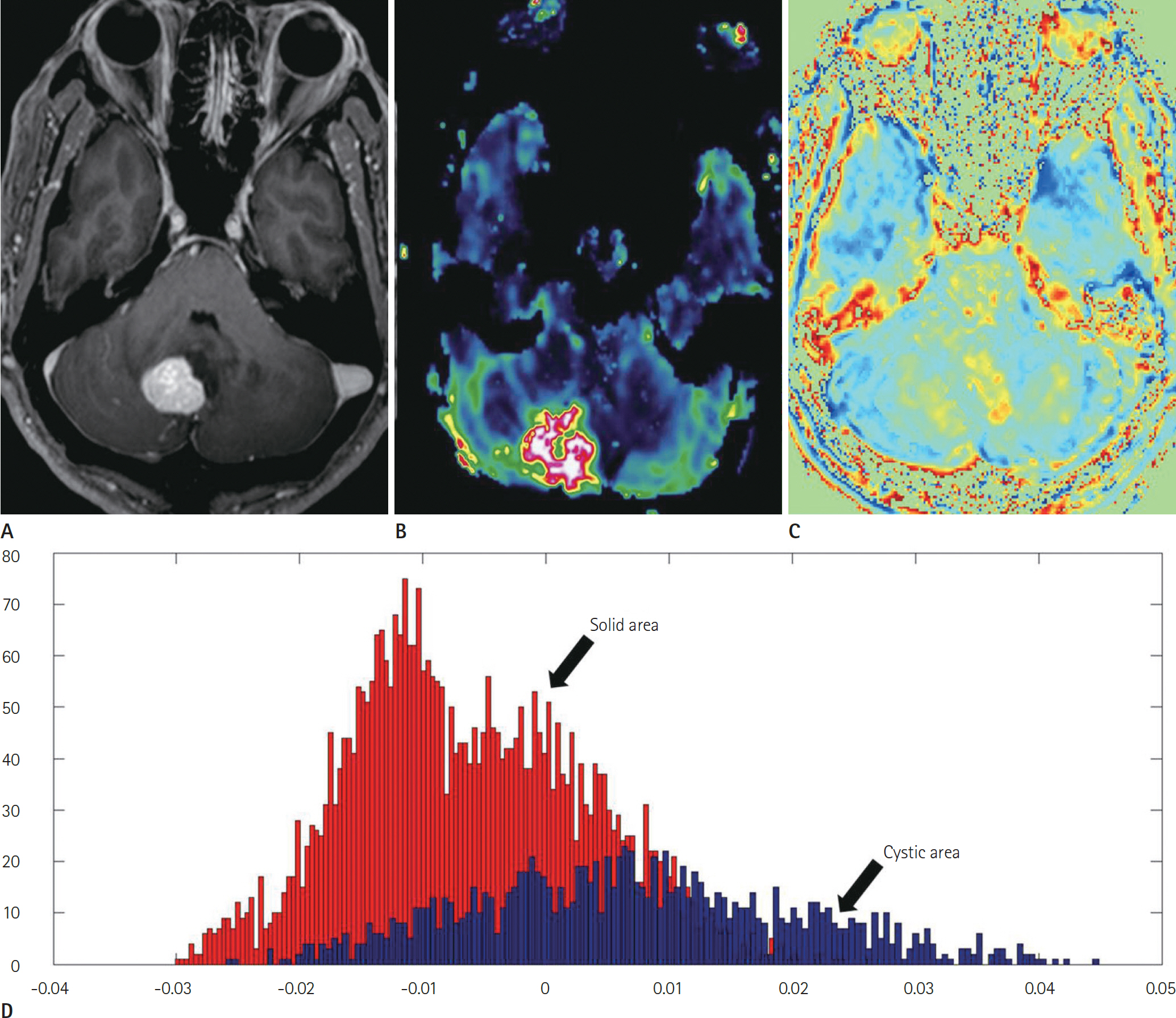
Article48. Shimizu H, Kumabe T, Shirane R, Yoshimoto T. Correlation between choline level measured by proton MR spectroscopy and Ki-67 labeling index in gliomas. AJNR Am J Neu-roradiol. 2000; 21:659–665.49. Park JE, Kim HS, Park KJ, Choi CG, Kim SJ. Histogram analysis of amide proton transfer imaging to identify contrast-enhancing low-grade brain tumor that mimics high-grade tumor: increased accuracy of MR perfusion. Radiology. 2015; 277:151–161.
Article50. Jiang S, Yu H, Wang X, Lu S, Li Y, Feng L, et al. Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 Tesla. Eur Radiol. 2016; 26:64–71.
Article51. Sagiyama K, Mashimo T, Togao O, Vemireddy V, Hatanpaa KJ, Maher EA, et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci U S A. 2014; 111:4542–4547.
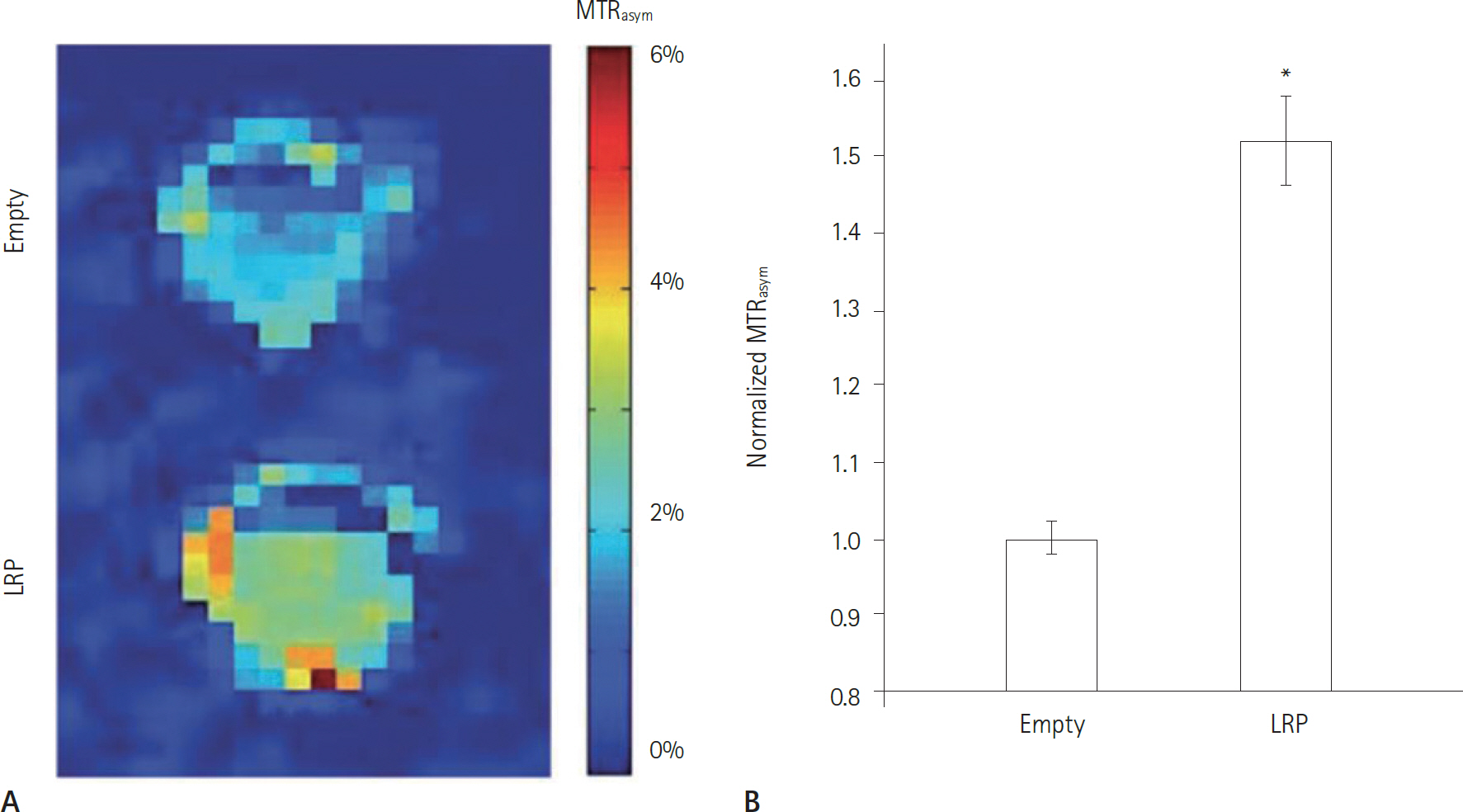
Article52. Ma B, Blakeley JO, Hong X, Zhang H, Jiang S, Blair L, et al. Applying amide proton transfer-weighted MRI to distin-guish pseudoprogression from true progression in malig-nant gliomas. J Magn Reson Imaging 2016 Jan 20 [Epub].http://dx.doi.org/10.1002/jmri.25159.53. Park KJ, Kim HS, Park JE, Shim WH, Kim SJ, Smith SA. Added value of amide proton transfer imaging to conven-tional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur Radiol 2016 Feb 16 [Epub].http://dx.doi.org/10.1007/s00330-016-4261-2.54. Farrar CT, Buhrman JS, Liu G, Kleijn A, Lamfers ML, McMa-hon MT, et al. Establishing the lysine-rich protein CEST re-porter gene as a CEST MR imaging detector for oncolytic virotherapy. Radiology. 2015; 275:746–754.
Article55. Choyke PL. Science to practice: monitoring oncolytic virus therapy with chemical exchange saturation transfer MR imaging–wishful thinking? Radiology. 2015; 275:625–626.
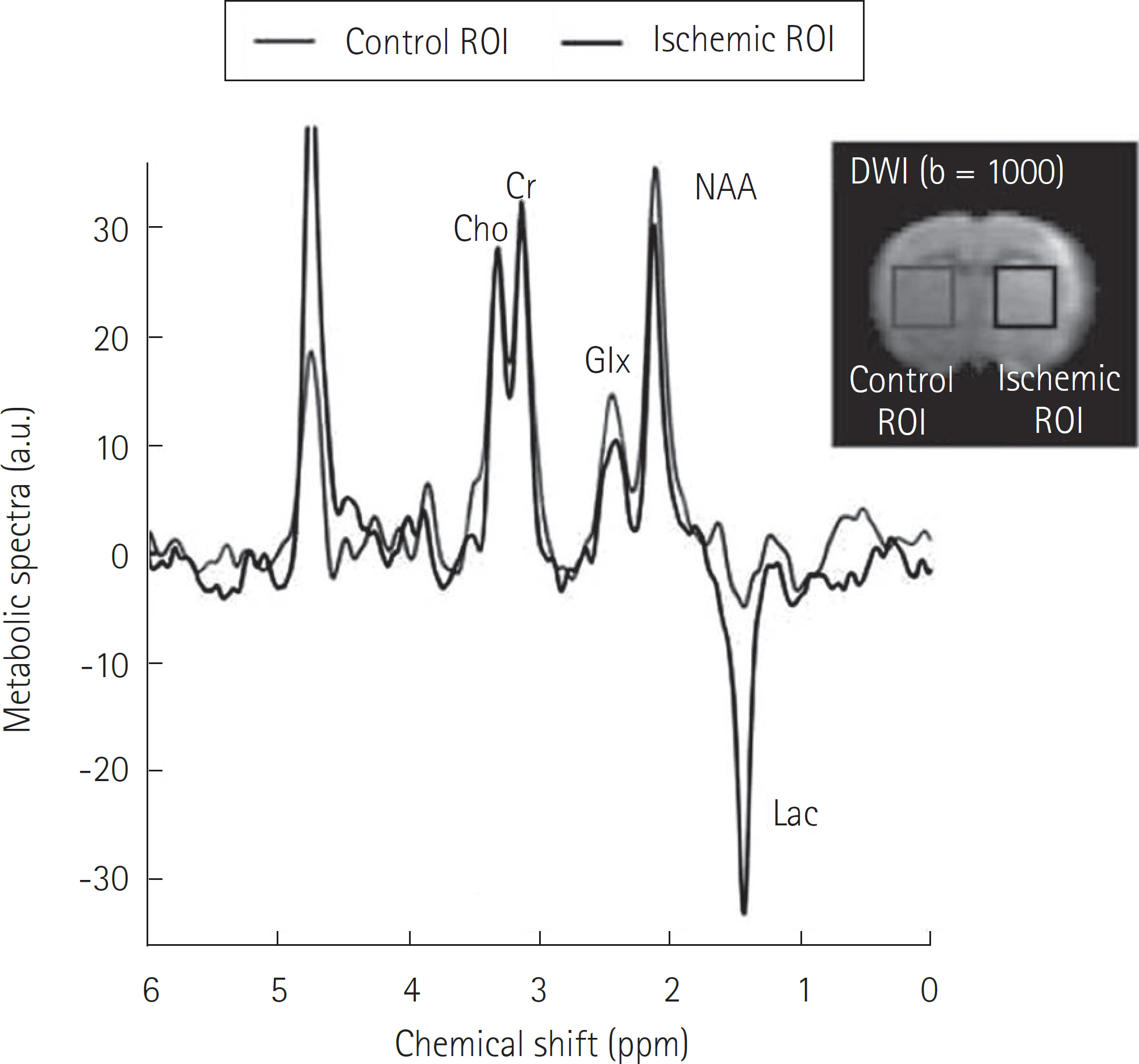
Article56. Sun PZ, Murata Y, Lu J, Wang X, Lo EH, Sorensen AG. Re-laxation-compensated fast multislice amide proton transfer (APT) imaging of acute ischemic stroke. Magn Reson Med. 2008; 59:1175–1182.
Article57. Sun PZ, Cheung JS, Wang E, Lo EH. Association between pH-weighted endogenous amide proton chemical exchange saturation transfer MRI and tissue lactic acidosis during acute ischemic stroke. J Cereb Blood Flow Metab. 2011; 31:1743–1750.
Article58. Sun PZ, Benner T, Copen WA, Sorensen AG. Early experience of translating pH-weighted MRI to image human subjects at 3 Tesla. Stroke. 2010; 41(10 Suppl):S147–S151.
Article59. Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson. 2000; 143:79–87.
Article60. Jin T, Wang P, Zong X, Kim SG. Magnetic resonance imaging of the Amine-Proton EXchange (APEX) dependent contrast. Neuroimage. 2012; 59:1218–1227.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Depiction of Acute Stroke Using 3-Tesla Clinical Amide Proton Transfer Imaging: Saturation Time Optimization Using an in vivo Rat Stroke Model, and a Preliminary Study in Human
- Emerging Techniques in Brain Tumor Imaging: What Radiologists Need to Know
- Proton therapy in pediatric brain tumors
- Current Applications and Future Perspectives of Brain Tumor Imaging
- Molecular Approaches for Brain Tumor Therapy;Gene Transfer and Anti-sense Oligonucleotides