Brain Tumor Res Treat.
2016 Oct;4(2):116-123. 10.14791/btrt.2016.4.2.116.
Palliative Resection of Metastatic Brain Tumors Previously Treated by Stereotactic Radiosurgery
- Affiliations
-
- 1Department of Neurosurgery, Konkuk University Medical Center, Seoul, Korea. kohyc@kuh.ac.kr
- 2Department of Pathology, Konkuk University Medical Center, Seoul, Korea.
- KMID: 2356980
- DOI: http://doi.org/10.14791/btrt.2016.4.2.116
Abstract
- BACKGROUND
Therapeutic approaches to brain metastases include surgery, whole-brain radiotherapy, stereotactic radiosurgery (SRS), and combination therapy. Recently, postoperative or preoperative SRS draws more attention to reduce postoperative recurrence in brain metastases. The goal of this study is to review surgical outcome of patients who had been treated by SRS, and to discuss the effectiveness of preoperative SRS.
METHODS
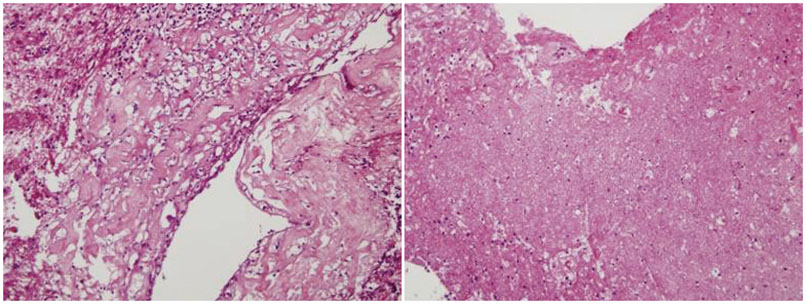
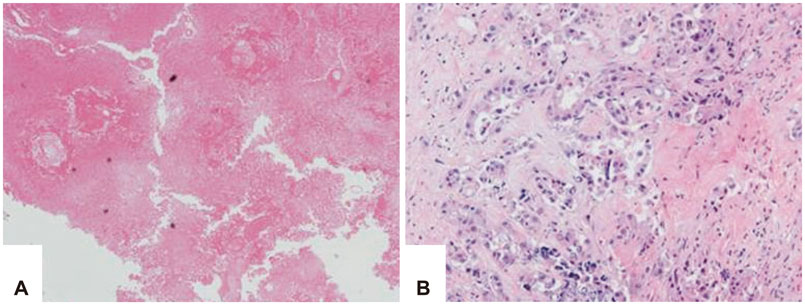
From 2009 to 2015, 174 patients were treated by SRS for brain metastases, and among these 50 patients underwent surgery. Eighteen patients underwent surgery after SRS, and 14 had oligometastases. The patients' median age at the time of surgery was 56 years (range, 34-84 years). The median follow-up duration was 16.5 months (range, 4-47 months). Pathological findings were classified as follows; radiation necrosis (Group I, n=3), mixed type (Group II, n=2), and tumor-dominant group (Group III, n=9). We compared surgical outcome in respect of steroid, mannitol dosage, Karnofsky performance scale, and pathological subgroups.
RESULTS
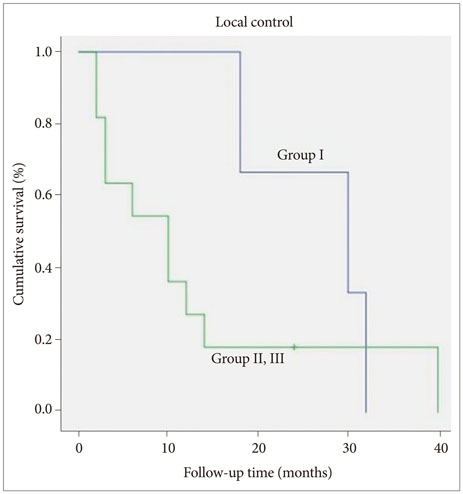
The median overall survival was 11 months (range, 2-40 months). Six, 12 and 24 months survival rate was 64.3, 42.9, and 28.6%, respectively. Improvement of Karnofsky performance score was achieved in 50% after surgery. The overall survival of Group I (26.6 months) was longer than the other groups (11.5 months). Additionally the patients were able to be weaned from medications, such as steroid administration after surgery was reduced in 10 cases, and mannitol dosage was reduced in 6 cases. Time interval within 3 months between SRS and surgery seemed to be related with better local control.
CONCLUSION
Surgical resection after radiologically and symptomatically progressed brain metastases previously treated with SRS seems to be effective in rapid symptom relief and provides an improvement in the quality of life. A short time interval between SRS and surgical resection seems to be associated with good local tumor control.
Keyword
MeSH Terms
Figure
Reference
-
1. Stelzer KJ. Epidemiology and prognosis of brain metastases. Surg Neurol Int. 2013; 4:Suppl 4. S192–S202.
Article2. Order SE, Hellman S, Von Essen CF, Kligerman MM. Improvement in quality of survival following whole-brain irradiation for brain metastasis. Radiology. 1968; 91:149–153.
Article3. Damiens K, Ayoub JP, Lemieux B, et al. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol. 2012; 19:254–258.
Article4. Pieper DR, Hess KR, Sawaya RE. Role of surgery in the treatment of brain metastases in patients with breast cancer. Ann Surg Oncol. 1997; 4:481–490.
Article5. Halasz LM, Uno H, Hughes M, et al. Comparative effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for patients with brain metastases from breast or non-small cell lung cancer. Cancer. 2016; 122:2091–2100.
Article6. Nieder C, Grosu AL, Gaspar LE. Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol. 2014; 9:155.
Article7. Videtic GM, Adelstein DJ, Mekhail TM, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for small-cell lung cancer-only brain metastases. Int J Radiat Oncol Biol Phys. 2007; 67:240–243.
Article8. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003; 29:533–540.
Article9. Baschnagel A, Wolters PL, Camphausen K. Neuropsychological testing and biomarkers in the management of brain metastases. Radiat Oncol. 2008; 3:26.
Article10. Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994; 28:797–802.
Article11. Patel U, Patel A, Cobb C, Benkers T, Vermeulen S. The management of brain necrosis as a result of SRS treatment for intra-cranial tumors. Transl Cancer Res. 2014; 3:373–382.12. Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery. 2010; 66:486–491. discussion 491-2.
Article13. Yaeger KA, Nair MN. Surgery for brain metastases. Surg Neurol Int. 2013; 4:Suppl 4. S203–S208.
Article14. Lunsford LD. Diagnosis and treatment of mass lesions using the Leksell stereotactic system. In : Lunsford LD, editor. Modern stereotactic neurosurgery. Boston: Martinus Nijhoff Publishing;1988. p. 145–168.15. Asher AL, Burri SH, Wiggins WF, et al. A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014; 88:899–906.
Article16. Patel KR, Burri SH, Asher AL, et al. Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery. 2016; 79:279–285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stereotactic radiosurgery for brain metastases
- How to use Leksell GammaPlan
- The Role of Stereotactic Radiosurgery in Metastasis to the Spine
- Stereotactic Radiosurgery for Metastatic Brain Tumor
- Comparative Analysis of Efficacy and Safety of Multisession Radiosurgery to Single Dose Radiosurgery for Metastatic Brain Tumors