J Korean Med Sci.
2016 Dec;31(12):1887-1896. 10.3346/jkms.2016.31.12.1887.
National Rules for Drug–Drug Interactions: Are They Appropriate for Tertiary Hospitals?
- Affiliations
-
- 1Nursing Department, Inha University, Incheon, Korea.
- 2The Center for Patient Safety Research and Practice, Division of General Internal Medicine, Brigham and Women’s Hospital, Boston, MA, USA. rufiji@gmail.com
- 3Harvard Medical School, Boston, MA, USA.
- 4Department of Emergency Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 5Department of Biomedical Informatics, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 6Seoul National University Hospital, Seoul, Korea.
- 7Seoul National University Bundang Hospital, Seongnam, Korea.
- 8Partners Healthcare Systems, Wellesley, MA, USA.
- KMID: 2355615
- DOI: http://doi.org/10.3346/jkms.2016.31.12.1887
Abstract
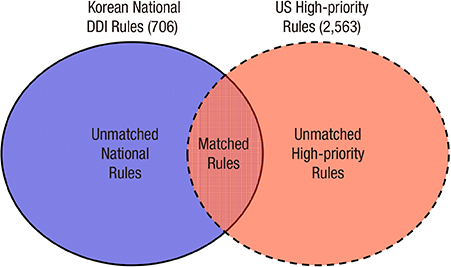
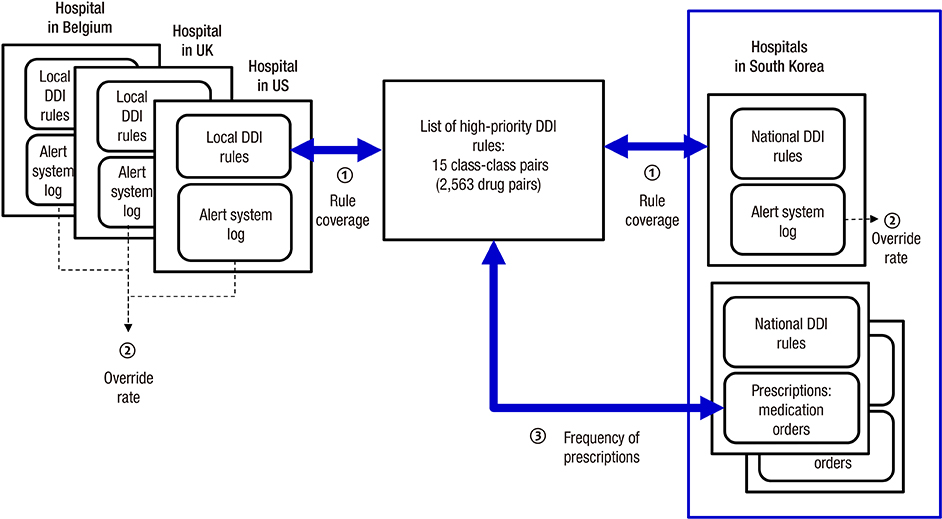
- The application of appropriate rules for drug-drug interactions (DDIs) could substantially reduce the number of adverse drug events. However, current implementations of such rules in tertiary hospitals are problematic as physicians are receiving too many alerts, causing high override rates and alert fatigue. We investigated the potential impact of Korean national DDI rules in a drug utilization review program in terms of their severity coverage and the clinical efficiency of how physicians respond to them. Using lists of high-priority DDIs developed with the support of the U.S. government, we evaluated 706 contraindicated DDI pairs released in May 2015. We evaluated clinical log data from one tertiary hospital and prescription data from two other tertiary hospitals. The measured parameters were national DDI rule coverage for high-priority DDIs, alert override rate, and number of prescription pairs. The coverage rates of national DDI rules were 80% and 3.0% at the class and drug levels, respectively. The analysis of the system log data showed an overall override rate of 79.6%. Only 0.3% of all of the alerts (n = 66) were high-priority DDI rules. These showed a lower override rate of 51.5%, which was much lower than for the overall DDI rules. We also found 342 and 80 unmatched high-priority DDI pairs which were absent in national rules in inpatient orders from the other two hospitals. The national DDI rules are not complete in terms of their coverage of severe DDIs. They also lack clinical efficiency in tertiary settings, suggesting improved systematic approaches are needed.
MeSH Terms
Figure
Reference
-
1. Choi NK, Park BJ. Strategy for establishing an effective Korean drug utilization review system. J Korean Med Assoc. 2010; 53:1130–1138.2. Slight SP, Seger DL, Nanji KC, Cho I, Maniam N, Dykes PC, Bates DW. Are we heeding the warning signs? Examining providers’ overrides of computerized drug-drug interaction alerts in primary care. PLoS One. 2013; 8:e85071.3. Park JY, Park KW. The contraindication of comedication drugs and drug utilization review. J Korean Med Assoc. 2012; 55:484–490.4. Jeon HL, Park JH, Kim DS, Choi BC. Review of the drug utilization review program of United States 47 States and suggestion of policy. Health Soc Welf Rev. 2014; 34:192–221.5. Shin HC, Park YT, Lee YT, Jo EC. Healthcare utilization monitoring system in Korea. Healthc Inform Res. 2015; 21:184–190.6. Phansalkar S, van der Sijs H, Tucker AD, Desai AA, Bell DS, Teich JM, Middleton B, Bates DW. Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013; 20:489–493.7. Cho I, Lee J, Han H, Phansalkar S, Bates DW. Evaluation of a Korean version of a tool for assessing the incorporation of human factors into a medication-related decision support system: the I-MeDeSA. Appl Clin Inform. 2014; 5:571–588.8. Cho I, Lee JH, Slight SP, Bates DW. International comparison of multi-hospital drug-drug interaction rules based on high and low priorities. In : Paper presented at the Spring Conference of the Korean Society of Medical Informatics(KOSMI); Seoul: The Korean Society of Medical Informatics;2014.9. Greenberg M, Ridgely MS. Clinical decision support and malpractice risk. JAMA. 2011; 306:90–91.10. Ahn EK, Cho SY, Shin D, Jang C, Park RW. Differences of reasons for alert overrides on contraindicated co-prescriptions by admitting department. Healthc Inform Res. 2014; 20:280–287.11. Lee SO, Je NK, Kim DS, Cheun BO, Hwang IO. Tiering ‘drug combinations to avoid’ and ‘drug-age precaution’ DUR alerts by severity level and its application. Yakhak Hoeji. 2015; 59:278–283.12. Paterno MD, Maviglia SM, Gorman PN, Seger DL, Yoshida E, Seger AC, Bates DW, Gandhi TK. Tiering drug-drug interaction alerts by severity increases compliance rates. J Am Med Inform Assoc. 2009; 16:40–46.13. Phansalkar S, Desai AA, Bell D, Yoshida E, Doole J, Czochanski M, Middleton B, Bates DW. High-priority drug-drug interactions for use in electronic health records. J Am Med Inform Assoc. 2012; 19:735–743.14. Bates DW. Clinical decision support and the law: the big picture. St Louis Univ J Health Law Policy. 2011; 5:319–324.15. van Roon EN, Flikweert S, le Comte M, Langendijk PN, Kwee-Zuiderwijk WJ, Smits P, Brouwers JR. Clinical relevance of drug-drug interactions : a structured assessment procedure. Drug Saf. 2005; 28:1131–1139.16. Böttiger Y, Laine K, Andersson ML, Korhonen T, Molin B, Ovesjö ML, Tirkkonen T, Rane A, Gustafsson LL, Eiermann B. SFINX-a drug-drug interaction database designed for clinical decision support systems. Eur J Clin Pharmacol. 2009; 65:627–633.17. Seidling HM, Storch CH, Bertsche T, Senger C, Kaltschmidt J, Walter-Sack I, Haefeli WE. Successful strategy to improve the specificity of electronic statin-drug interaction alerts. Eur J Clin Pharmacol. 2009; 65:1149–1157.18. Smithburger PL, Kane-Gill SL, Benedict NJ, Falcione BA, Seybert AL. Grading the severity of drug-drug interactions in the intensive care unit: a comparison between clinician assessment and proprietary database severity rankings. Ann Pharmacother. 2010; 44:1718–1724.19. Smith WD, Hatton RC, Fann AL, Baz MA, Kaplan B. Evaluation of drug interaction software to identify alerts for transplant medications. Ann Pharmacother. 2005; 39:45–50.20. Helmons PJ, Suijkerbuijk BO, Nannan Panday PV, Kosterink JG. Drug-drug interaction checking assisted by clinical decision support: a return on investment analysis. J Am Med Inform Assoc. 2015; 22:764–772.21. Jang C, Yoo KB, Kim W, Park MY, Ahn EK, Park RW. New alert override codes for the drug utilization review system derived from outpatient prescription data from a tertiary teaching hospital in Korea. Healthc Inform Res. 2016; 22:39–45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Basic Principles of Drug Interaction
- Drug-drug Interactions between Psychotropic Agents and Other Drugs in Physically Ill Patients: Experience of Consultation-liason in Korea University Hospital
- Inhibition of Cytochrome P450 Enzymes by Drugs—Molecular Basis and Practical Applications
- Antidepressants and Related Drug Interactions
- Drug-Drug Interactions: Mood Stabilizers and Anti-Anxiety Drugs