Immune Netw.
2016 Oct;16(5):261-270. 10.4110/in.2016.16.5.261.
Original Antigenic Sin Response to RNA Viruses and Antiviral Immunity
- Affiliations
-
- 1Department of Microbiology, The Institute of Viral Diseases, College of Medicine, Korea University, Seoul 02841, Korea. ms0392@korea.ac.kr
- KMID: 2355019
- DOI: http://doi.org/10.4110/in.2016.16.5.261
Abstract
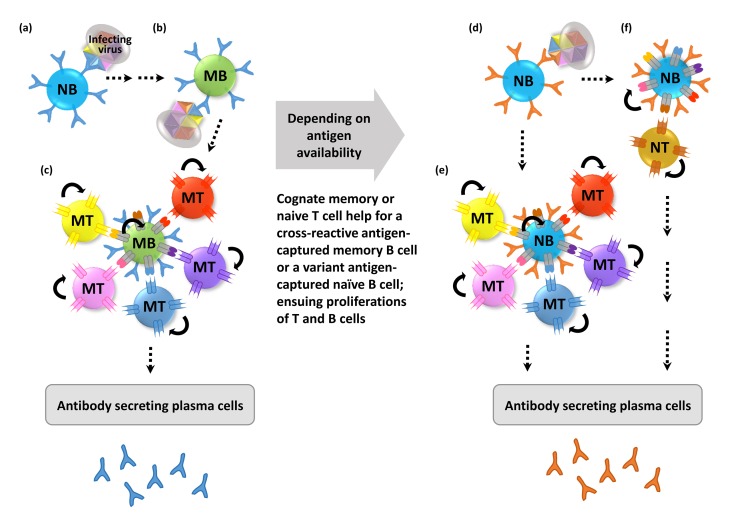
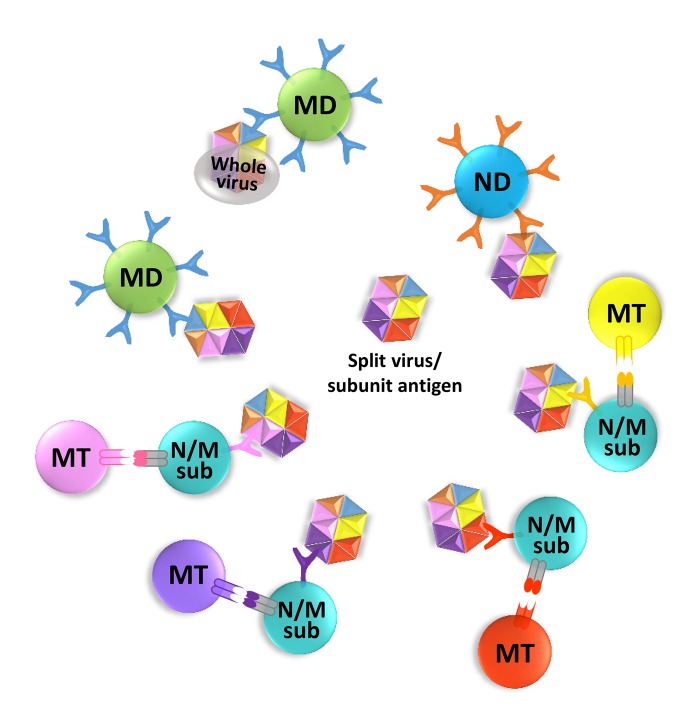
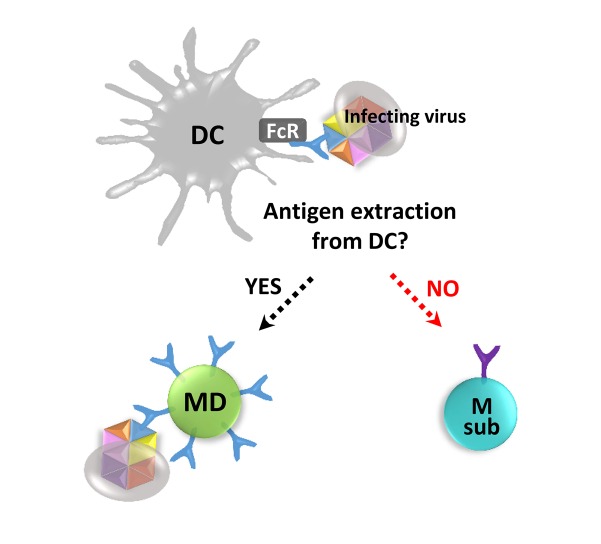
- The human immune system has evolved to fight against foreign pathogens. It plays a central role in the body's defense mechanism. However, the immune memory geared to fight off a previously recognized pathogen, tends to remember an original form of the pathogen when a variant form subsequently invades. This has been termed 'original antigenic sin'. This adverse immunological effect can alter vaccine effectiveness and sometimes cause enhanced pathogenicity or additional inflammatory responses, according to the type of pathogen and the circumstances of infection. Here we aim to give a simplified conceptual understanding of virus infection and original antigenic sin by comparing and contrasting the two examples of recurring infections such as influenza and dengue viruses in humans.
Keyword
Figure
Reference
-
1. Davenport FM, Hennessy AV, Francis T Jr. Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J Exp Med. 1953; 98:641–656. PMID: 13109114.
Article2. Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJ, Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev. 2015; 268:340–364. PMID: 26497532.
Article3. McHeyzer-Williams M, Okitsu S, Wang N, Heyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol. 2012; 12:24–34.
Article4. Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O'Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science. 2016; 352:aaf1098. PMID: 27102489.
Article5. Okhrimenko A, Grun JR, Westendorf K, Fang Z, Reinke S, von RP, Wassilew G, Kuhl AA, Kudernatsch R, Demski S, Scheibenbogen C, Tokoyoda K, McGrath MA, Raftery MJ, Schonrich G, Serra A, Chang HD, Radbruch A, Dong J. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci U S A. 2014; 111:9229–9234. PMID: 24927527.
Article6. Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011; 11:532–543. PMID: 21760609.
Article8. Tangye SG, Avery DT, Deenick EK, Hodgkin PD. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J Immunol. 2003; 170:686–694. PMID: 12517929.
Article9. Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992; 360:264–265. PMID: 1436108.
Article10. Swain SL. CD4 T-cell memory can persist in the absence of class II. Philos Trans R Soc Lond B Biol Sci. 2000; 355:407–411. PMID: 10794062.
Article11. Hale JS, Ahmed R. Memory T follicular helper CD4 T cells. Front Immunol. 2015; 6:16. PMID: 25699040.
Article12. Schmidlin H, Diehl SA, Blom B. New insights into the regulation of human B-cell differentiation. Trends Immunol. 2009; 30:277–285. PMID: 19447676.
Article13. Benson MJ, Erickson LD, Gleeson MW, Noelle RJ. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr Opin Immunol. 2007; 19:275–280. PMID: 17433651.
Article14. Lees JR, Farber DL. Generation, persistence and plasticity of CD4 T-cell memories. Immunology. 2010; 130:463–470. PMID: 20465569.
Article15. Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012; 209:1241–1253. PMID: 22753927.
Article16. Eisen HN. Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol Res. 2014; 2:381–392. PMID: 24795350.
Article17. Farrington LA, Smith TA, Grey F, Hill AB, Snyder CM. Competition for antigen at the level of the APC is a major determinant of immunodominance during memory inflation in murine cytomegalovirus infection. J Immunol. 2013; 190:3410–3416. PMID: 23455500.
Article18. Johansson BE, Kilbourne ED. Immunization with dissociated neuraminidase, matrix, and nucleoproteins from influenza A virus eliminates cognate help and antigenic competition. Virology. 1996; 225:136–144. PMID: 8918540.
Article19. Pham GH, Iglesias BV, Gosselin EJ. Fc receptor-targeting of immunogen as a strategy for enhanced antigen loading, vaccination, and protection using intranasally administered antigen-pulsed dendritic cells. Vaccine. 2014; 32:5212–5220. PMID: 25068496.
Article20. Krammer F, Palese P, Steel J. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol. 2015; 386:301–321. PMID: 25007847.
Article21. Nachbagauer R, Wohlbold TJ, Hirsh A, Hai R, Sjursen H, Palese P, Cox RJ, Krammer F. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J Virol. 2014; 88:13260–13268. PMID: 25210189.
Article22. Adar Y, Singer Y, Levi R, Tzehoval E, Perk S, Banet-Noach C, Nagar S, Arnon R, Ben-Yedidia T. A universal epitope-based influenza vaccine and its efficacy against H5N1. Vaccine. 2009; 27:2099–2107. PMID: 19356612.
Article23. Hemann EA, Kang SM, Legge KL. Protective CD8 T cell-mediated immunity against influenza A virus infection following influenza virus-like particle vaccination. J Immunol. 2013; 191:2486–2494. PMID: 23885108.
Article24. Zeng W, Tan AC, Horrocks K, Jackson DC. A lipidated form of the extracellular domain of influenza M2 protein as a self-adjuvanting vaccine candidate. Vaccine. 2015; 33:3526–3532. PMID: 26049002.
Article25. Gibbons RV. Dengue conundrums. Int J Antimicrob Agents. 2010; 36(Suppl 1):S36–S39. PMID: 20696556.
Article26. Weiskopf D, Sette A. T-cell immunity to infection with dengue virus in humans. Front Immunol. 2014; 5:93. PMID: 24639680.
Article27. McKittrick N, Frank I, Jacobson JM, hite CJW, Kim D, Kappes R, DiGiorgio C, Kenney T, Boyer J, Tebas P. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med. 2013; 158:19–26. PMID: 23277897.28. Berger CT, Greiff V, Mehling M, Fritz S, Meier MA, Hoenger G, Conen A, Recher M, Battegay M, Reddy ST, Hess C. Influenza vaccine response profiles are affected by vaccine preparation and preexisting immunity, but not HIV infection. Hum Vaccin Immunother. 2015; 11:391–396. PMID: 25692740.
Article29. Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010; 33:451–463. PMID: 21029957.
Article30. Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013; 11:153. PMID: 23800265.
Article31. Johns MC, Eick AA, Blazes DL, Lee SE, Perdue CL, Lipnick R, Vest KG, Russell KL, DeFraites RF, Sanchez JL. Seasonal influenza vaccine and protection against pandemic (H1N1) 2009-associated illness among US military personnel. PLoS One. 2010; 5:e10722. PMID: 20502705.
Article32. Eick-Cost AA, Tastad KJ, Guerrero AC, Johns MC, Lee SE, Macintosh VH, Burke RL, Blazes DL, Russell KL, Sanchez JL. Effectiveness of seasonal influenza vaccines against influenza-associated illnesses among US military personnel in 2010-11: a case-control approach. PLoS One. 2012; 7:e41435. PMID: 22859985.
Article33. Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005; 23:503–514. PMID: 16286018.
Article34. Batista FD, Neuberger MS. B cells extract and present immobilized antigen: implications for affinity discrimination. EMBO J. 2000; 19:513–520. PMID: 10675320.
Article35. Faix DJ, Hawksworth AW, Myers CA, Hansen CJ, Ortiguerra RG, Halpin R, Wentworth D, Pacha LA, Schwartz EG, Garcia SM, Eick-Cost AA, Clagett CD, Khurana S, Golding H, Blair PJ. Decreased serologic response in vaccinated military recruits during 2011 correspond to genetic drift in concurrent circulating pandemic A/H1N1 viruses. PLoS One. 2012; 7:e34581. PMID: 22514639.
Article36. Skowronski DM, Janjua NZ, De SG, Sabaiduc S, Eshaghi A, Dickinson JA, Fonseca K, Winter AL, Gubbay JB, Krajden M, Petric M, Charest H, Bastien N, Kwindt TL, Mahmud SM, Van CP, Li Y. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One. 2014; 9:e92153. PMID: 24667168.
Article37. Chambers BS, Parkhouse K, Ross TM, Alby K, Hensley SE. Identification of hemagglutinin residues responsible for H3N2 antigenic drift during the 2014-2015 influenza season. Cell Rep. 2015; 12:1–6. PMID: 26119736.
Article38. Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz JM. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009; 361:1945–1952. PMID: 19745214.
Article39. Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del GG, Rappuoli R. Influenza vaccine immunology. Immunol Rev. 2011; 239:167–177. PMID: 21198671.
Article40. Neumann G, Kawaoka Y. The first influenza pandemic of the new millennium. Influenza Other Respir. Viruses. 2011; 5:157–166.41. Tan GS, Leon PE, Albrecht RA, Margine I, Hirsh A, Bahl J, Krammer F. Broadly-reactive neutralizing and non-neutralizing antibodies directed against the H7 influenza virus hemagglutinin reveal divergent mechanisms of protection. PLoS Pathog. 2016; 12:e1005578. PMID: 27081859.
Article42. Viboud C, Eisenstein J, Reid AH, Janczewski TA, Morens DM, Taubenberger JK. Age- and sex-specific mortality associated with the 1918-1919 influenza pandemic in Kentucky. J Infect Dis. 2013; 207:721–729. PMID: 23230061.
Article43. Reed C, Chaves SS, Perez A, D'Mello T, Daily KP, Aragon D, Meek JI, Farley MM, Ryan P, Lynfield R, Morin CA, Hancock EB, Bennett NM, Zansky SM, Thomas A, Lindegren ML, Schaffner W, Finelli L. Complications among adults hospitalized with influenza: a comparison of seasonal influenza and the 2009 H1N1 pandemic. Clin Infect Dis. 2014; 59:166–174. PMID: 24785230.
Article44. Morens DM, Taubenberger JK, Folkers GK, Fauci AS. Pandemic influenza's 500th anniversary. Clin Infect Dis. 2010; 51:1442–1444. PMID: 21067353.
Article45. Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, Cox NJ, Katz JM, Taubenberger JK, Palese P, Garcia-Sastre A. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005; 310:77–80. PMID: 16210530.
Article46. Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol. 1998; 160:322–327. PMID: 9551987.47. Zaiss DM, Boog CJ, van EW, Sijts AJ. Considerations in the design of vaccines that induce CD8 T cell mediated immunity. Vaccine. 2010; 28:7716–7722. PMID: 20851090.
Article48. Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999; 286:1377–1381. PMID: 10558996.
Article49. Shane HL, Klonowski KD. Every breath you take: the impact of environment on resident memory CD8 T cells in the lung. Front Immunol. 2014; 5:320. PMID: 25071780.
Article50. Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994; 152:1653–1661. PMID: 8120375.51. Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014; 95:215–224. PMID: 24006506.
Article52. Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, Swayne DE, Katze MG. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006; 443:578–581. PMID: 17006449.
Article53. Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008; 3:499–522. PMID: 18039138.
Article54. Schmid MA, Diamond MS, Harris E. Dendritic cells in dengue virus infection: targets of virus replication and mediators of immunity. Front Immunol. 2014; 5:647. PMID: 25566258.
Article55. Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998; 161:6338–6346. PMID: 9834124.56. Dalrymple N, Mackow ER. Productive dengue virus infection of human endothelial cells is directed by heparan sulfate-containing proteoglycan receptors. J Virol. 2011; 85:9478–9485. PMID: 21734047.
Article57. Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004; 41:173–183. PMID: 15159063.
Article58. Dalrymple NA, Mackow ER. Virus interactions with endothelial cell receptors: implications for viral pathogenesis. Curr Opin Virol. 2014; 7:134–140. PMID: 25063986.
Article59. Thomas KA, Valenzuela NM, Reed EF. The perfect storm: HLA antibodies, complement, FcgammaRs, and endothelium in transplant rejection. Trends Mol Med. 2015; 21:319–329. PMID: 25801125.60. Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010; 8:271–283. PMID: 20833378.
Article61. Short KR, Veldhuis Kroeze EJ, Reperant LA, Richard M, Kuiken T. Influenza virus and endothelial cells: a species specific relationship. Front Microbiol. 2014; 5:653. PMID: 25520707.
Article62. Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, Maskin B, Bugna J, Rubinstein C, Aguilar L, Dalurzo L, Libster R, Savy V, Baumeister E, Aguilar L, Cabral G, Font J, Solari L, Weller KP, Johnson J, Echavarria M, Edwards KM, Chappell JD, Crowe JE Jr, Williams JV, Melendi GA, Polack FP. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med. 2011; 17:195–199. PMID: 21131958.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inflammasomes in antiviral immunity: clues for influenza vaccine development
- Towards the Application of Human Defensins as Antivirals
- COVID-19: Balancing between Transmission Suppression and Immunity
- Sensing DNA Viruses and Bacteria by Intracellular DNA Sensors
- Synergistic effect of ribavirin and vaccine for protection during early infection stage of foot-and-mouth disease